
Product of the Clemmensen’s reduction is-
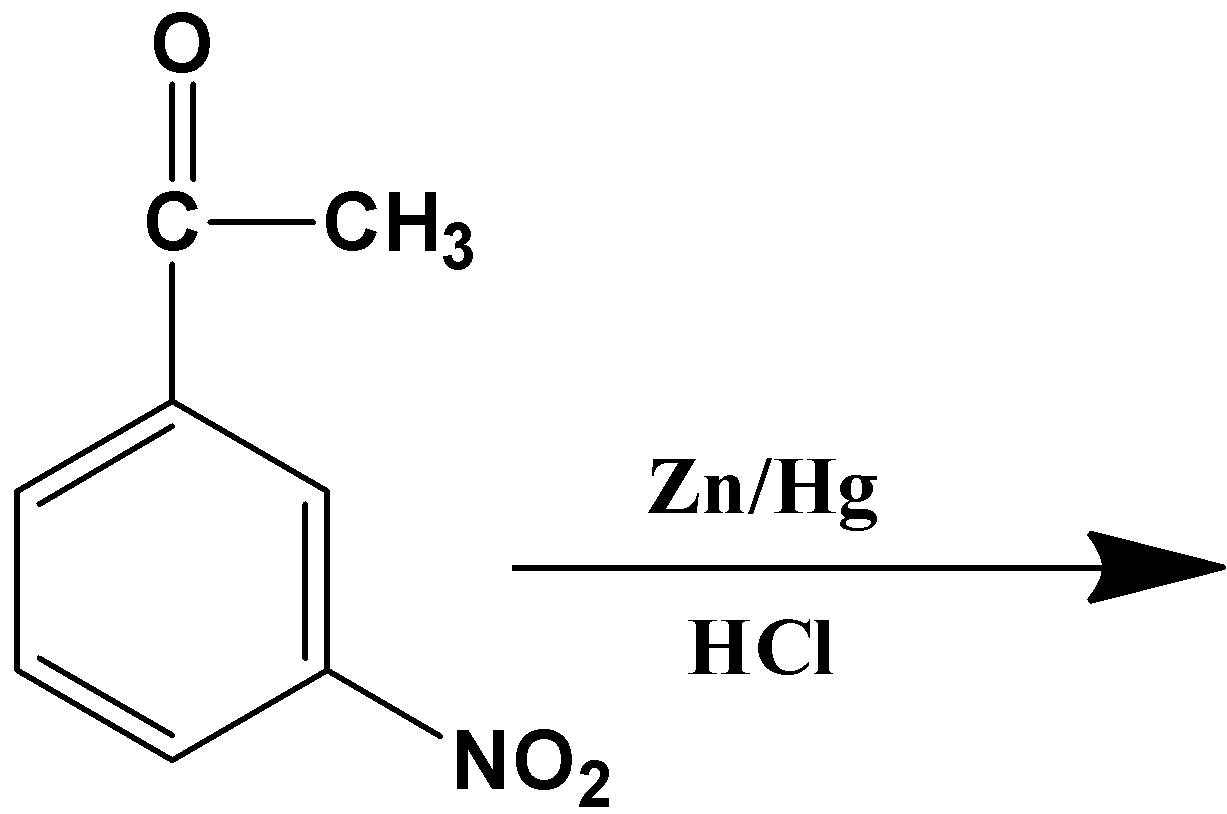
(A)
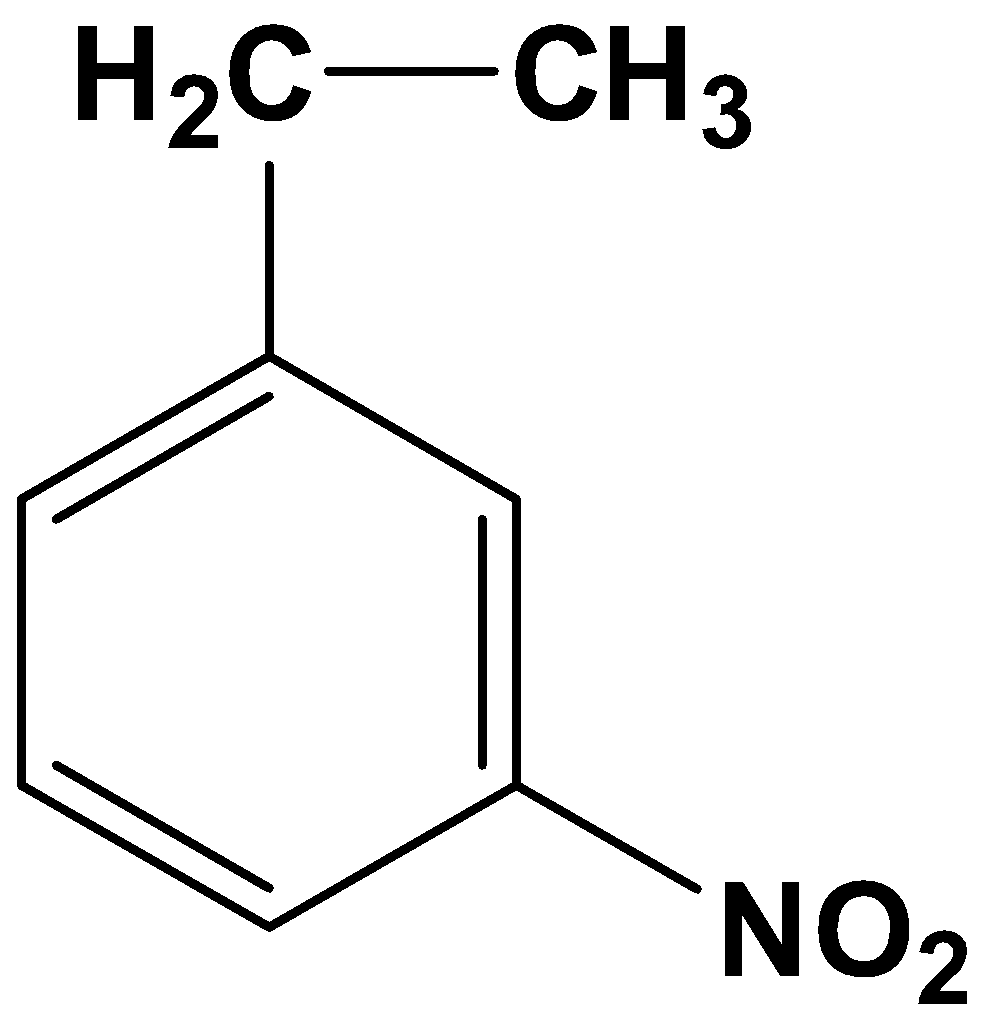
(B)
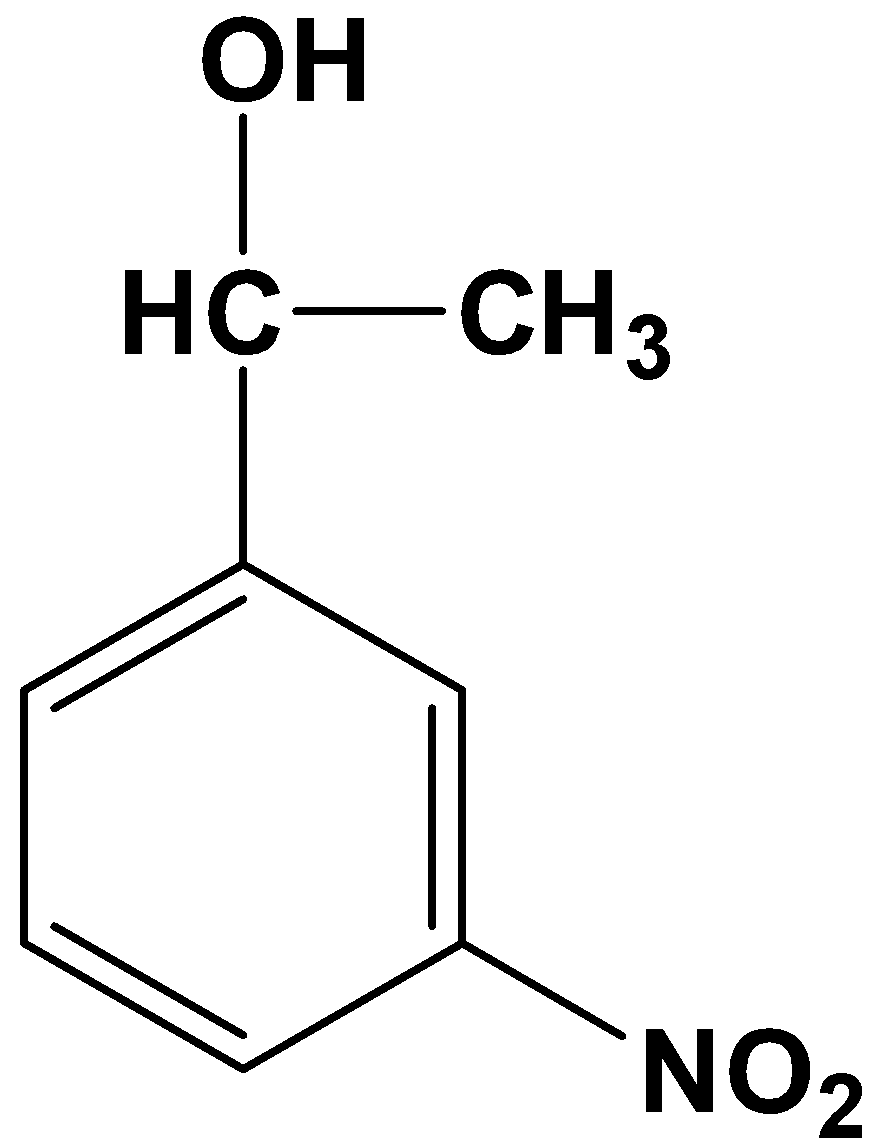
(C)
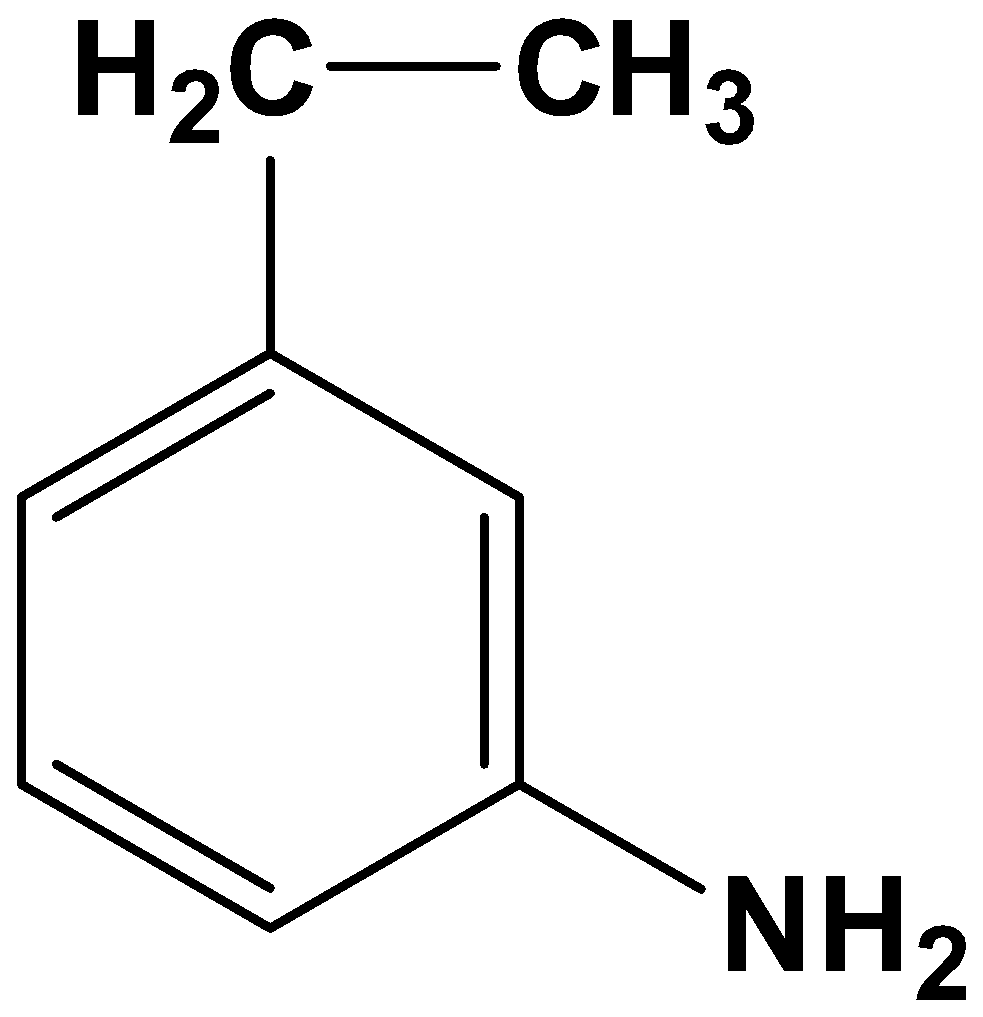
(D)
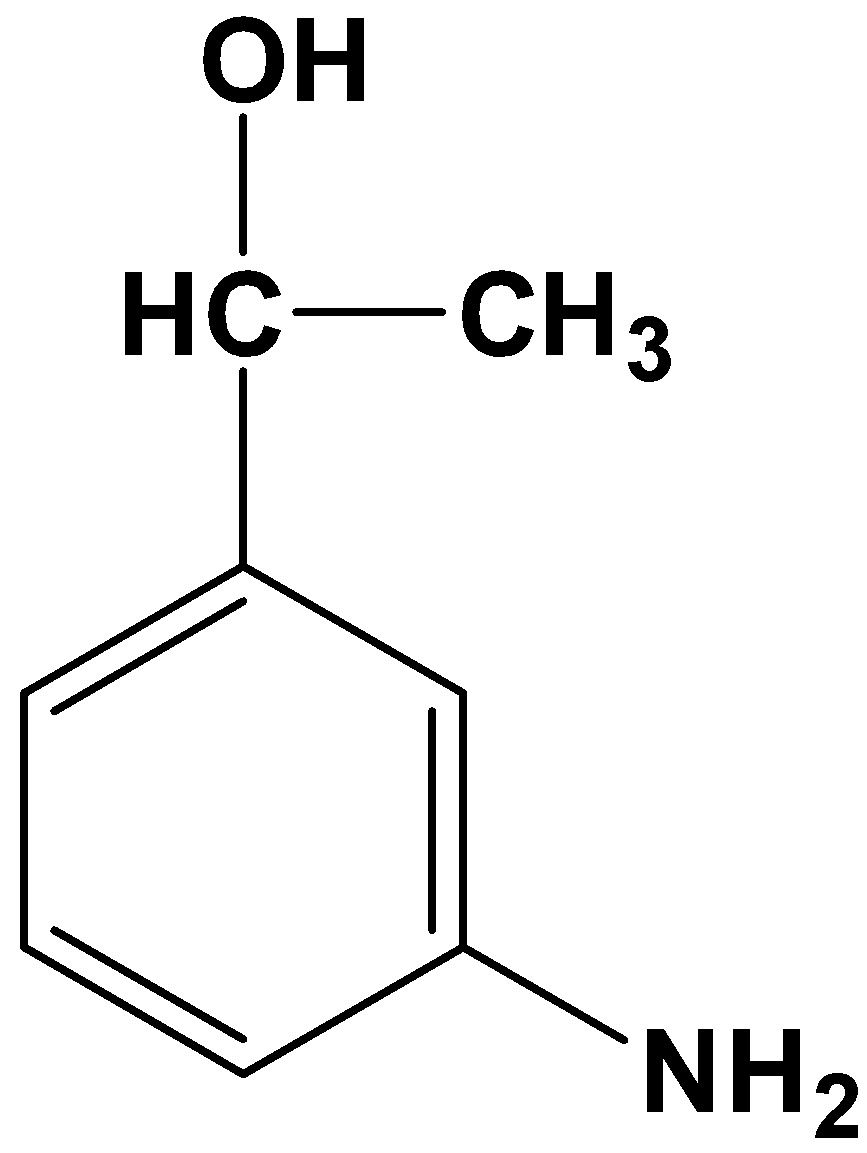





Answer
578.1k+ views
Hint: This reaction is very specific in nature. It reduces the carbonyl carbon to methyl groups.
Complete Solution :
The name of the chemical reaction is already given as Clemmensen’s reduction. This is named after a Danish chemist who goes by Erik Christian Clemmensen.
It is very popular for its specificity and less complicated procedures that have to be carried out in the laboratory for the formation of products. As it is very clear, the reaction in overall is a reduction process where addition of hydrogen atoms takes place. The reagents only target carbonyl carbons and reduce them to alkane groups.
The general formula of the reaction is as follows:
- For aldehyde
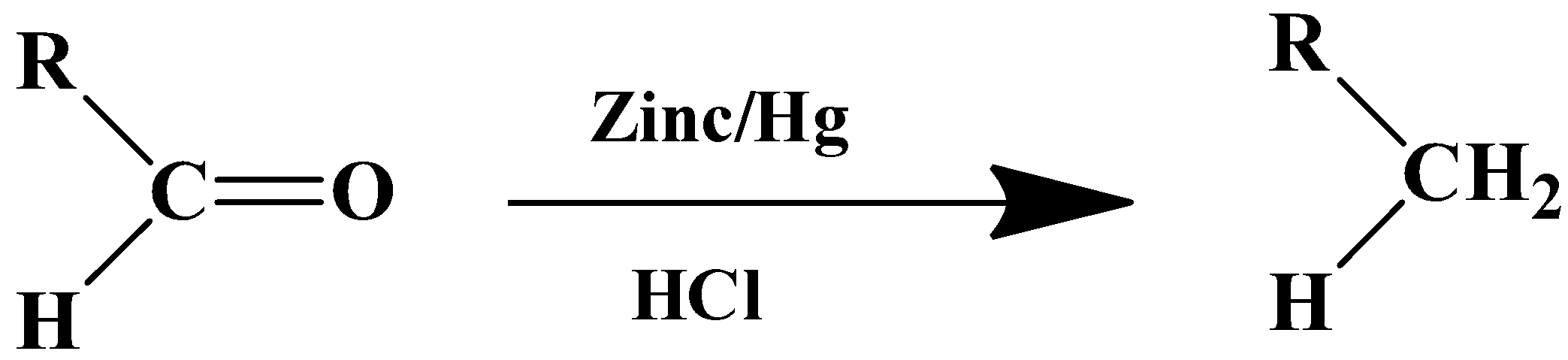
- For ketones
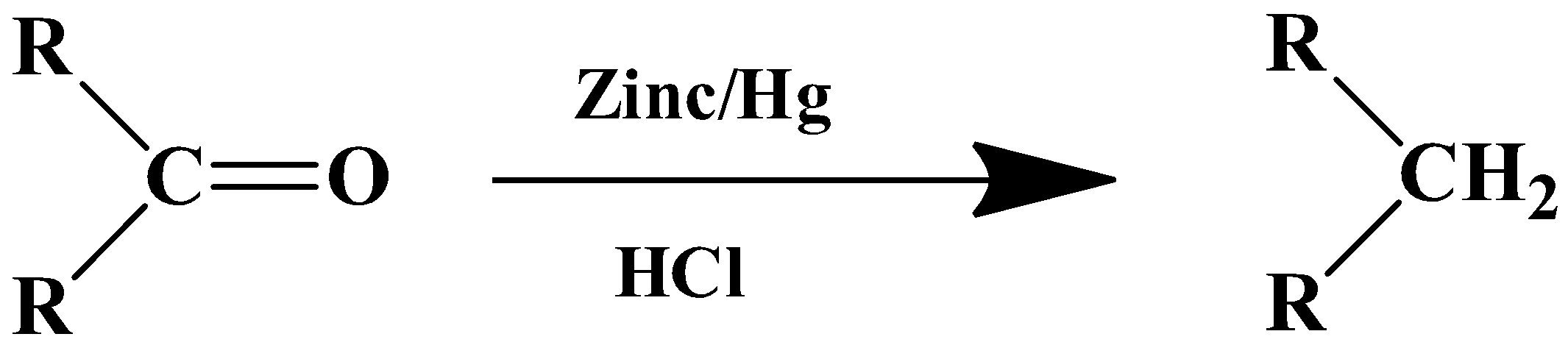
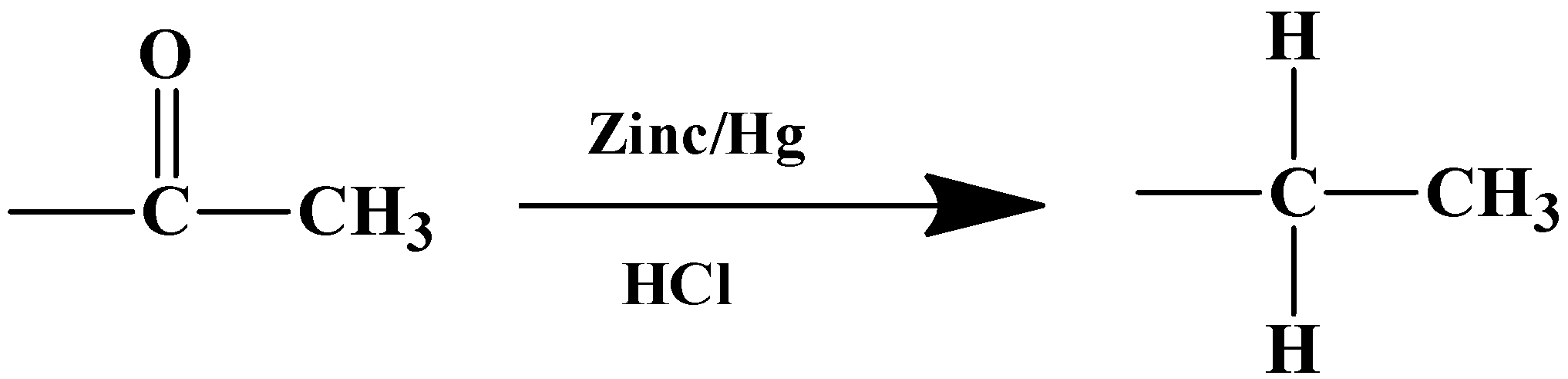
The mechanism of this reaction is boosted by the metal catalyst. Zinc’s surface is used as the reaction centre. The solvent in this case is a strong inorganic acid- $HCl$. The substrate therefore has to be stable in a highly acidic medium.
- Let’s get down to the product that will form from the reaction given in the question. The given substrate has only one carbonyl compound which is a part of the acyl group ($-COC{{H}_{3}}$) placed at the meta-position relative to the nitro group ($-N{{O}_{2}}$). Owing to the specificity of the reagents, only the carbonyl carbon in the acyl group will be reduced to its corresponding alkane, which means:
The product therefore is option (A)-
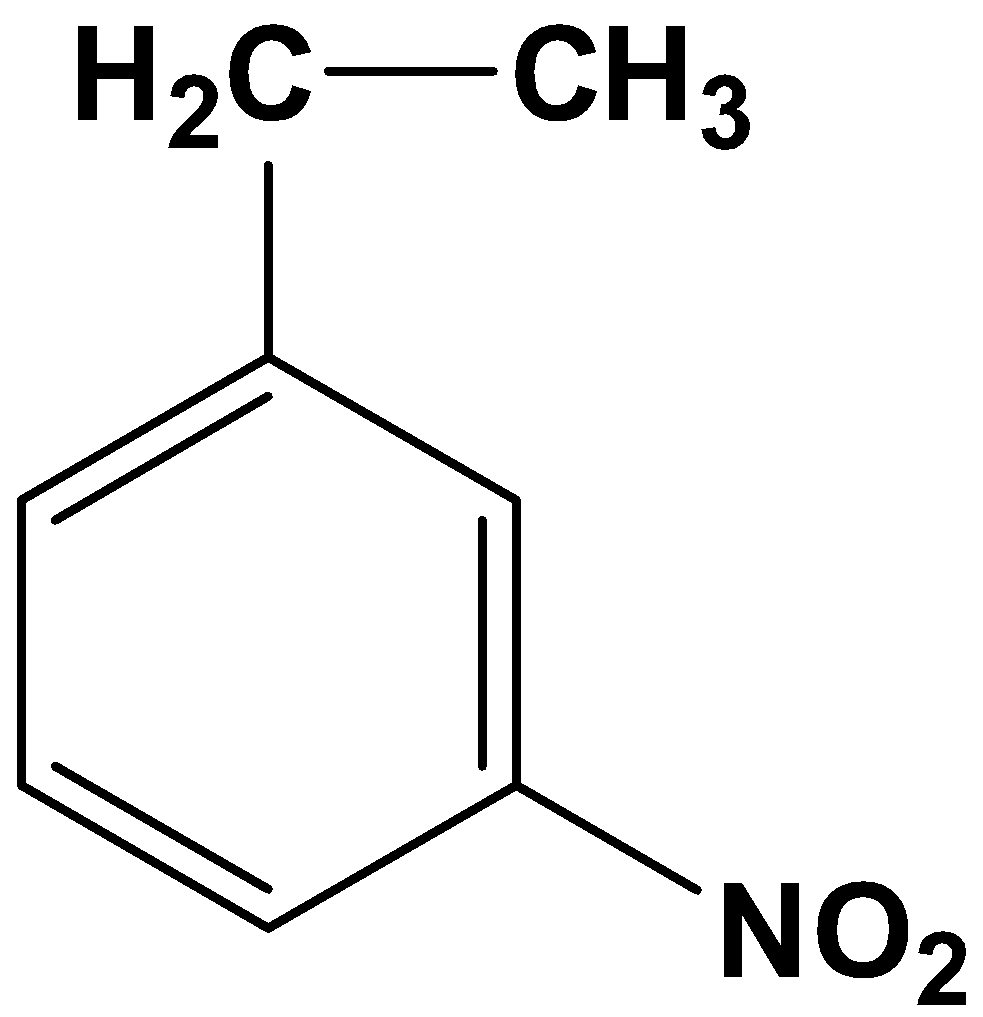
Now let us talk about the other options:
- Option (B)
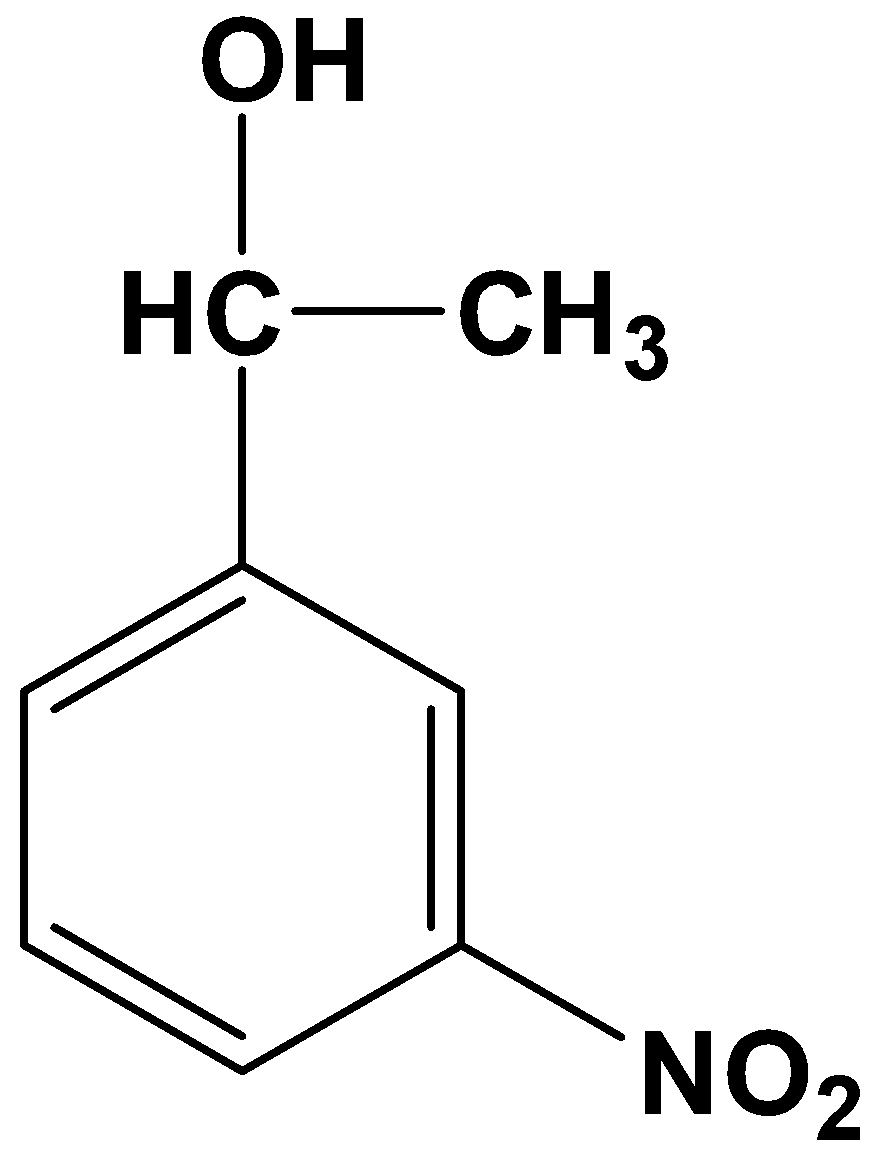
This is not possible because it is a one-step reduction where a carbonyl compound reduces only to its alcohol counterpart. Clemmensen’s reduction involves strong reducing agents which directly convert a carbonyl compound into its alkane form.
- Option (C)
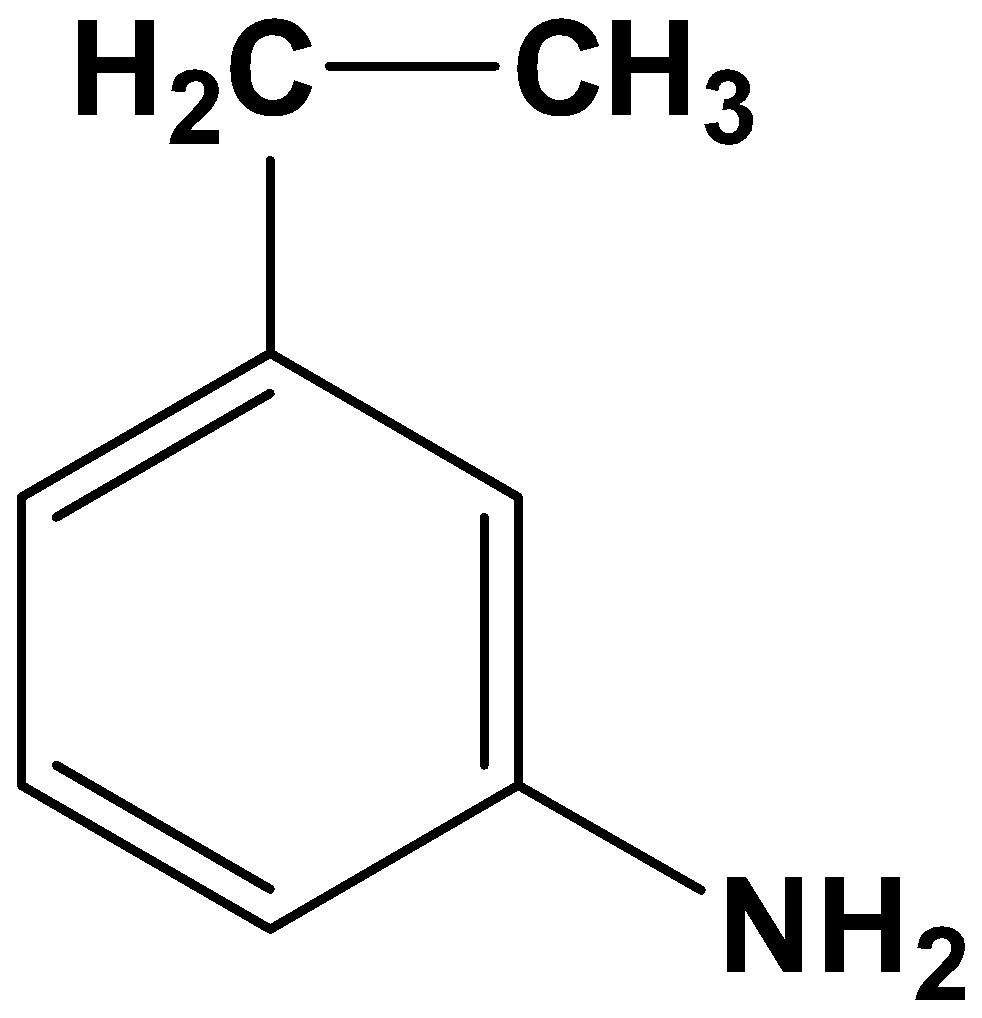
Here both the acyl group and nitro group have been reduced. As the reagents are very specific this is not possible.
- Option (D)
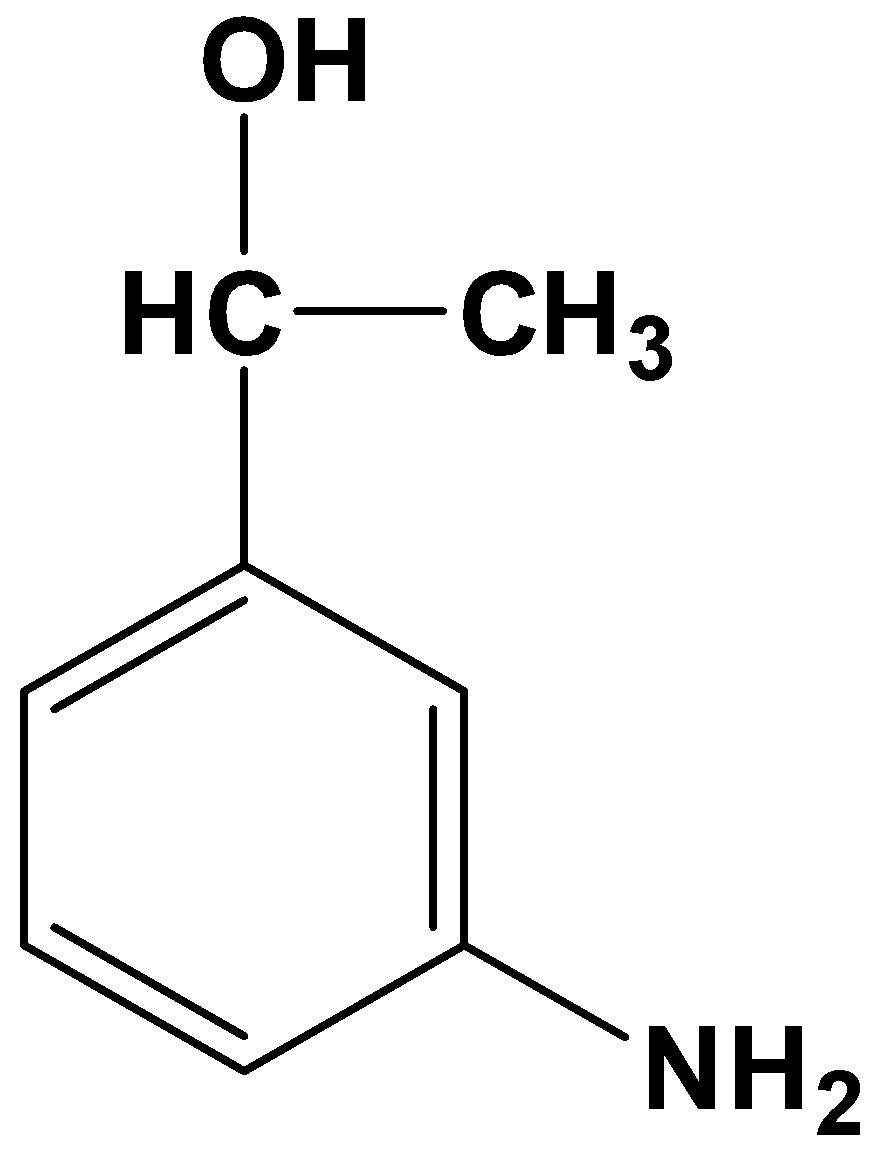
This product is totally absurd because it reduces the acyl and nitro group to various degrees. Therefore, it is not possible.
So, the correct answer is “Option A”.
Note: Care should be taken to not confuse this with wolff-kishner reduction. It may achieve the same results but it is through a totally different mechanism. The products which are not stable in highly acidic medium used in Clemmensen’s reduction are tried with the wolff-kishner process as this has a basic medium.
Complete Solution :
The name of the chemical reaction is already given as Clemmensen’s reduction. This is named after a Danish chemist who goes by Erik Christian Clemmensen.
It is very popular for its specificity and less complicated procedures that have to be carried out in the laboratory for the formation of products. As it is very clear, the reaction in overall is a reduction process where addition of hydrogen atoms takes place. The reagents only target carbonyl carbons and reduce them to alkane groups.
The general formula of the reaction is as follows:
- For aldehyde

- For ketones
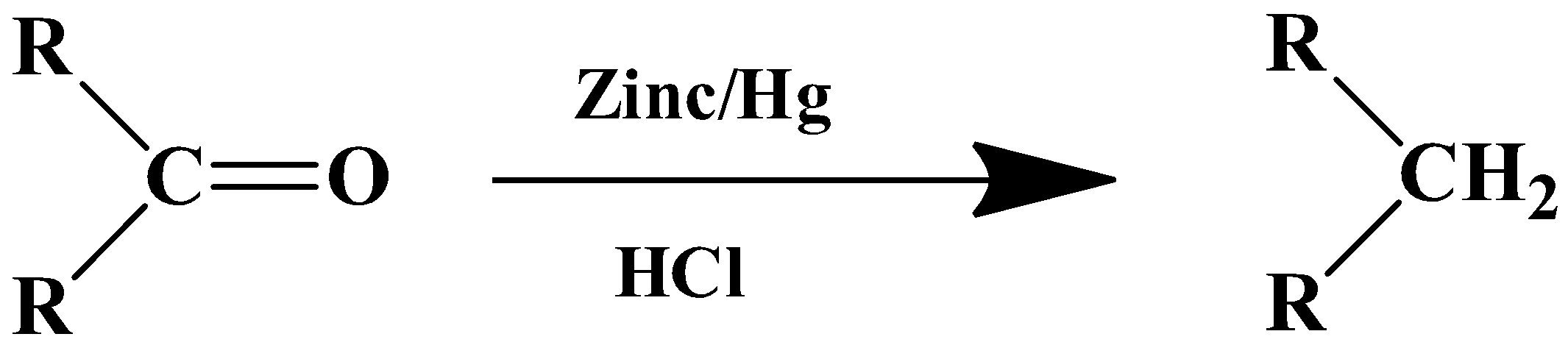
The mechanism of this reaction is boosted by the metal catalyst. Zinc’s surface is used as the reaction centre. The solvent in this case is a strong inorganic acid- $HCl$. The substrate therefore has to be stable in a highly acidic medium.
- Let’s get down to the product that will form from the reaction given in the question. The given substrate has only one carbonyl compound which is a part of the acyl group ($-COC{{H}_{3}}$) placed at the meta-position relative to the nitro group ($-N{{O}_{2}}$). Owing to the specificity of the reagents, only the carbonyl carbon in the acyl group will be reduced to its corresponding alkane, which means:

The product therefore is option (A)-

Now let us talk about the other options:
- Option (B)

This is not possible because it is a one-step reduction where a carbonyl compound reduces only to its alcohol counterpart. Clemmensen’s reduction involves strong reducing agents which directly convert a carbonyl compound into its alkane form.
- Option (C)

Here both the acyl group and nitro group have been reduced. As the reagents are very specific this is not possible.
- Option (D)

This product is totally absurd because it reduces the acyl and nitro group to various degrees. Therefore, it is not possible.
So, the correct answer is “Option A”.
Note: Care should be taken to not confuse this with wolff-kishner reduction. It may achieve the same results but it is through a totally different mechanism. The products which are not stable in highly acidic medium used in Clemmensen’s reduction are tried with the wolff-kishner process as this has a basic medium.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Coming together federation is practiced in A India class 12 social science CBSE

How was the Civil Disobedience Movement different from class 12 social science CBSE




