
What is the product in the following reaction?
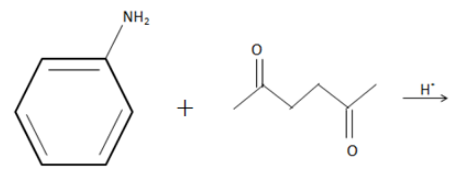
(A)
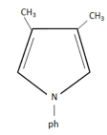
(B)
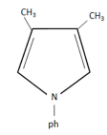
(C)
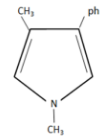
(D)





Answer
572.1k+ views
Hint:One of the given compounds contains a primary amine and the other contains two ketone groups. This primary amine group can easily react with one of the oxygen atoms in the ketone group and eliminate one water molecule. Further check whether the formed intermediate is stable and any requirement of rearrangement.
Complete answer:We have an \[ - N{H_2}\] group in aniline and two ketone groups in $2,5 - diketone$ .
The $ - N{H_2}$ group can actively react with one of the ketone groups to eliminate water and form a primary imine as an intermediate. An imine is a $ - C = N - R$ , here $R$ is our phenyl group.
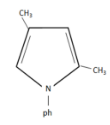
We can rearrange this intermediate to eliminate the other oxygen and form a cyclic ring containing $N - ph$ group. This results in the formation of a pyrrole. From this, we can understand that this is a condensation reaction.
The condensation of aniline with $2,5 - hexanedione$ to form $2,5 - dimethyl - 1 - phenylpyrrole$ is an example of Paal-Knorr synthesis.
We can write an equation for this reaction as follows:
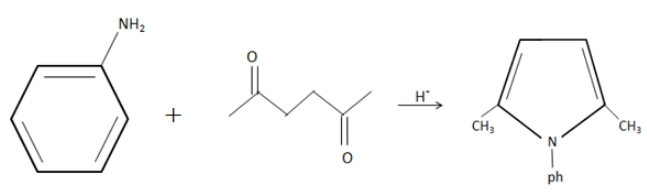
Additional information: Paal-Knorr Pyrrole Synthesis is the condensation of a 1,4-dicarbonyl compound with an excess amount of a primary amine or ammonia to produce a pyrrole. This reaction generates furans, pyrroles and thiophenes.
Therefore the product formed in our given reaction is $2,5 - dimethyl - 1 - phenylpyrrole$ i.e, option D.
Note:The given reaction is an example for Paal-Knorr synthesis. In this reaction, we are using a primary aniline group to react with a diketone to form a pyrrole. This reaction occurs in an acidic medium and the product formed in this reaction is \[2,5 - dimethyl - 1 - phenylpyrrole\] .
Complete answer:We have an \[ - N{H_2}\] group in aniline and two ketone groups in $2,5 - diketone$ .
The $ - N{H_2}$ group can actively react with one of the ketone groups to eliminate water and form a primary imine as an intermediate. An imine is a $ - C = N - R$ , here $R$ is our phenyl group.
We can rearrange this intermediate to eliminate the other oxygen and form a cyclic ring containing $N - ph$ group. This results in the formation of a pyrrole. From this, we can understand that this is a condensation reaction.
The condensation of aniline with $2,5 - hexanedione$ to form $2,5 - dimethyl - 1 - phenylpyrrole$ is an example of Paal-Knorr synthesis.
We can write an equation for this reaction as follows:

Additional information: Paal-Knorr Pyrrole Synthesis is the condensation of a 1,4-dicarbonyl compound with an excess amount of a primary amine or ammonia to produce a pyrrole. This reaction generates furans, pyrroles and thiophenes.
Therefore the product formed in our given reaction is $2,5 - dimethyl - 1 - phenylpyrrole$ i.e, option D.
Note:The given reaction is an example for Paal-Knorr synthesis. In this reaction, we are using a primary aniline group to react with a diketone to form a pyrrole. This reaction occurs in an acidic medium and the product formed in this reaction is \[2,5 - dimethyl - 1 - phenylpyrrole\] .
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




