
What is the product formed when acetyl chloride reacts with aniline in the presence of pyridine?
(A) Acetanilide
(B) Acetic anhydride
(C) Benzoylacetate
(D) Benzanilide
Answer
590.1k+ views
Hint: Reaction of acetyl chloride with aniline in presence of pyridine is Nucleophiles substitution of aromatic amines with acid. It forms N-substituted amides. In nucleophilic substitution nucleophile is substituted by other nucleophile
Step by step answer: Aniline is Aromatic amines in which amino group is attached to the benzene ring.
In the reaction of acetyl chloride $C{H_3}COCl$ with aniline, H-atom of $ - N{H_2}$ group is replaced by acetyl group $[C{H_3}C{O^ - }]$ therefore this is a type of nucleophilic substitution.
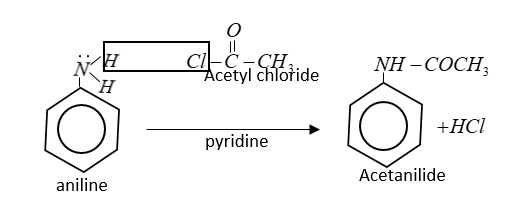
In this reaction $HCl$is lost which reacts with pyridine to form hydrochloride salt of pyridine.
This reaction is also known as Schotten Baumann reaction.
Role pyridine in reaction: Pyridine is a nucleophile for carbonyl groups. It is used as a catalyst in acylation reactions.
N-atoms in pyridine are nucleophilic because the lone pair of electrons on nitrogen cannot be delocalized around the ring.
Mechanism:
(i) Mechanism includes regular attack of nucleophile of an amine on a carbonyl.
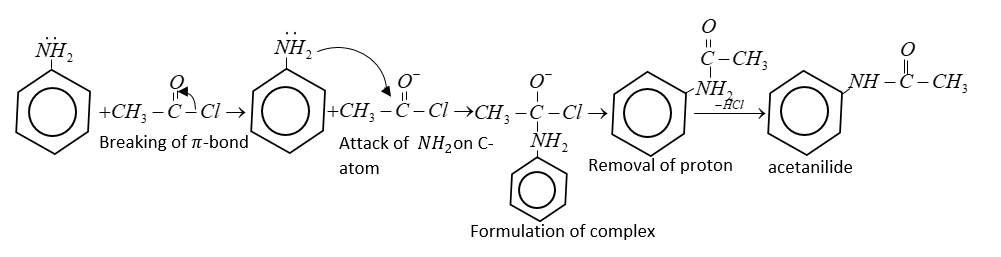
In the final step proton is accepted from $ - N{H_2}$ and from acetanilide.
Therefore, from the above explanation the correct option is (A) Acetanilide.
Note: N-acetylation of aniline with acetyl chloride leads to production of one equivalent acid $(HCl),$ which is formed from salt with unreacted pyridine.
If we add an equivalent base to neutralize $HCl,$ catalytic amount of pyridine yields more in a shorter period.
Step by step answer: Aniline is Aromatic amines in which amino group is attached to the benzene ring.

In the reaction of acetyl chloride $C{H_3}COCl$ with aniline, H-atom of $ - N{H_2}$ group is replaced by acetyl group $[C{H_3}C{O^ - }]$ therefore this is a type of nucleophilic substitution.

In this reaction $HCl$is lost which reacts with pyridine to form hydrochloride salt of pyridine.
This reaction is also known as Schotten Baumann reaction.
Role pyridine in reaction: Pyridine is a nucleophile for carbonyl groups. It is used as a catalyst in acylation reactions.
N-atoms in pyridine are nucleophilic because the lone pair of electrons on nitrogen cannot be delocalized around the ring.
Mechanism:
(i) Mechanism includes regular attack of nucleophile of an amine on a carbonyl.

In the final step proton is accepted from $ - N{H_2}$ and from acetanilide.
Therefore, from the above explanation the correct option is (A) Acetanilide.
Note: N-acetylation of aniline with acetyl chloride leads to production of one equivalent acid $(HCl),$ which is formed from salt with unreacted pyridine.
If we add an equivalent base to neutralize $HCl,$ catalytic amount of pyridine yields more in a shorter period.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




