
How will you prepare methoxy methane from
(A) Methyl bromide
(B) Diazomethane
(C) Methyl alcohol
Answer
585.6k+ views
Hint: Methoxy methane (dimethyl ether) is the organic compound with the formula $C{{H}_ {3}} OC{{H}_ {3}} $, simplified to ${{C}_ {2}} {{H}_ {6}} O$. The simplest ether, it is a colourless gas that is a useful precursor to other organic compounds and an aerosol propellant that is currently being demonstrated for use in a variety of fuel applications. It is an isomer of ethanol.
Complete step by step solution:
We have been provided methyl bromide, diazomethane and methyl alcohol.
We need to obtain methoxy methane (dimethyl ether) from these three compounds,
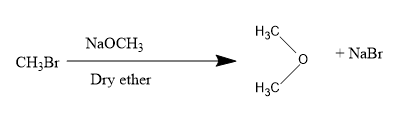
So, firstly we have methyl bromide: $C{{H}_ {3}} OH$$C{{H}_ {3}} Br$
For converting into methoxy methane we will be treating methyl bromide with dry ether along with $NaOC{{H}_ {3}} $,
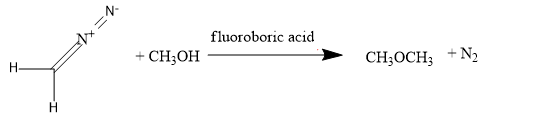
Next, we have diazomethane: $N\equiv N-C{{H}_ {2}} $
For, converting into methoxy methane, add the ethyl alcohol along with the diazomethane. By treating the ethyl alcohol with the diazomethane in the presence of the fluoroboric acid, methoxy methane will be formed,
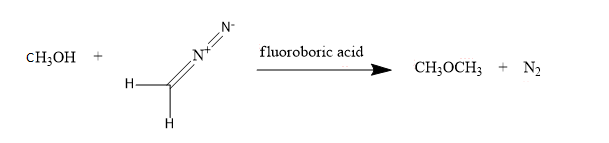
Next, we have methyl alcohol: $C{{H}_ {3}} OH$,
For converting into methoxy methane, diazomethane is added in methyl alcohol in the presence of fluoroboric acid,
So, these are the conversions of methyl bromide, diazomethane and methyl alcohol into methoxy methane.
Note: It is used as an aerosol propellant, as a refrigerant, and as a blowing agent for the production of some foams. It can also be used as a fuel in diesel engines. Ethyl methyl ether, or methoxy ethane, is a colourless gas at room temperature, having a boiling point of 7.6 degree Celsius. Like dimethyl ether, it is fairly water soluble.
Complete step by step solution:
We have been provided methyl bromide, diazomethane and methyl alcohol.
We need to obtain methoxy methane (dimethyl ether) from these three compounds,
So, firstly we have methyl bromide: $C{{H}_ {3}} OH$$C{{H}_ {3}} Br$
For converting into methoxy methane we will be treating methyl bromide with dry ether along with $NaOC{{H}_ {3}} $,

Next, we have diazomethane: $N\equiv N-C{{H}_ {2}} $
For, converting into methoxy methane, add the ethyl alcohol along with the diazomethane. By treating the ethyl alcohol with the diazomethane in the presence of the fluoroboric acid, methoxy methane will be formed,

Next, we have methyl alcohol: $C{{H}_ {3}} OH$,
For converting into methoxy methane, diazomethane is added in methyl alcohol in the presence of fluoroboric acid,

So, these are the conversions of methyl bromide, diazomethane and methyl alcohol into methoxy methane.
Note: It is used as an aerosol propellant, as a refrigerant, and as a blowing agent for the production of some foams. It can also be used as a fuel in diesel engines. Ethyl methyl ether, or methoxy ethane, is a colourless gas at room temperature, having a boiling point of 7.6 degree Celsius. Like dimethyl ether, it is fairly water soluble.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




