
How will you prepare:
A.Propiophenone from propanenitrile
B.4-chlorobenzaldehyde from 4-chlorotoluene
Write the structure and give IUPAC name of the following:
A.$\beta - {\text{hydroxybutyraldehyde}}$
B.Isobutyraldehyde
C.Isopropyl methyl ketone.
Answer
544.8k+ views
Hint: The first conversion that is the conversion of propanenitrile to propiophenone is carried out using the Grignard reagent and water. The acid anhydride followed by hydrolysis leads to the formation of the aldehyde.
Complete step-by-step answer:To prepare Propiophenone from propanenitrile:
We take the help of Grignard reagent in the above conversion. We are using Grignard reagent so that we can introduce the phenyl group which is the final product for this we have taken phenyl bromide magnesium. The reaction occurs as follow:
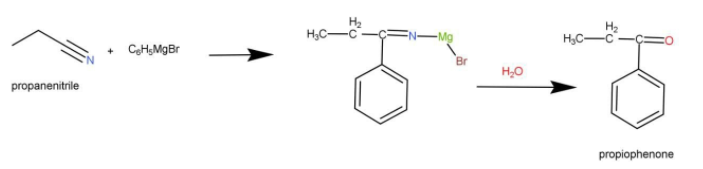
To prepare 4-chlorobenzaldehyde from 4-chlorotoluene:
This is a very simple conversion. We will just require the use of the anhydride; we are using acetic anhydride to convert the 4-chlorotoluene into 4-chlorobenzaldehyde. The reaction occurs as:
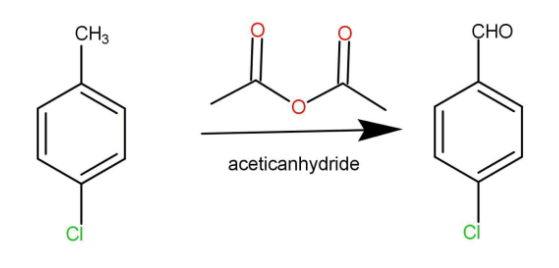
Now we will write the IUPAC names of the given common names:
$\beta - {\text{hydroxybutyraldehyde}}$
The term “but” indicates that there are 4 carbon atoms present in the parent carbon chain. The beta position is the second position with respect to the carbonyl functional group. As the common name suggests the functional group present is aldehyde. Hence the structure and the IUPAC name will be:
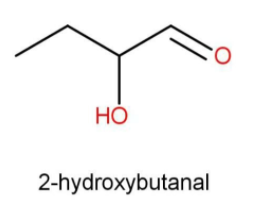
While writing IUPAC names we use “al” as a suffix for the aldehyde functional group. The side substituent such as hydroxy is written first along with its position with respect to the function.
Isobutyraldehyde
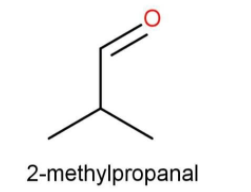
Here also the functional group is aldehyde. The number of carbons is also 4. The iso group is present to the aldehyde. While writing the IUPAC name again the side substituent that is methyl is written first.
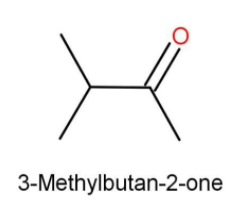
Isopropyl methyl ketone
Here the functional group is ketone. On one side there is methyl and on the other side there is isopropyl group. While writing the IUPAC name for ketone the position for ketone functional group is also defined. The longest linear chain formed is of 4 carbons and hence we used “but”. The methyl is present as a side substituent on carbon number 3.
Note: IUPAC stands for International Union for Pure and Applied Chemistry. Grignard reagent is basically alkyl halide magnesium. It is an organometallic reagent that is those which have metal carbon bonds.
Complete step-by-step answer:To prepare Propiophenone from propanenitrile:
We take the help of Grignard reagent in the above conversion. We are using Grignard reagent so that we can introduce the phenyl group which is the final product for this we have taken phenyl bromide magnesium. The reaction occurs as follow:

To prepare 4-chlorobenzaldehyde from 4-chlorotoluene:
This is a very simple conversion. We will just require the use of the anhydride; we are using acetic anhydride to convert the 4-chlorotoluene into 4-chlorobenzaldehyde. The reaction occurs as:

Now we will write the IUPAC names of the given common names:
$\beta - {\text{hydroxybutyraldehyde}}$
The term “but” indicates that there are 4 carbon atoms present in the parent carbon chain. The beta position is the second position with respect to the carbonyl functional group. As the common name suggests the functional group present is aldehyde. Hence the structure and the IUPAC name will be:

While writing IUPAC names we use “al” as a suffix for the aldehyde functional group. The side substituent such as hydroxy is written first along with its position with respect to the function.
Isobutyraldehyde
Here also the functional group is aldehyde. The number of carbons is also 4. The iso group is present to the aldehyde. While writing the IUPAC name again the side substituent that is methyl is written first.

Isopropyl methyl ketone
Here the functional group is ketone. On one side there is methyl and on the other side there is isopropyl group. While writing the IUPAC name for ketone the position for ketone functional group is also defined. The longest linear chain formed is of 4 carbons and hence we used “but”. The methyl is present as a side substituent on carbon number 3.

Note: IUPAC stands for International Union for Pure and Applied Chemistry. Grignard reagent is basically alkyl halide magnesium. It is an organometallic reagent that is those which have metal carbon bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




