
What is the preparation method of white phosphorus?
Answer
515.1k+ views
Hint: Phosphorus is a chemical element that has the molecular symbol P. Phosphorus comes in a wide variety of allotropes; the most famous of these are the red and white phosphorus. Allotropes are different variations of a particular chemical substance that have the capacity to exist as the same physical forms.
Complete answer:
In nature, there are various allotropic sources of phosphorus.
The various forms result from the various ways atoms can be bound together. White phosphorus is one such allotrope that exists for phosphorus.
The white phosphorus allotrope would be a waxy solid that explodes or burns readily and is used in chemical processing or in smoke explosives.
White phosphorus (yellow phosphorus) also known as tetraphosphorus (${P_4}$) occur as tetrahedral molecules that are comprised of four atoms of the same kind. The molecule is said to be made up of six independent single P–P bonds.
The figure showing the structure of a white phosphorous is illustrated below:
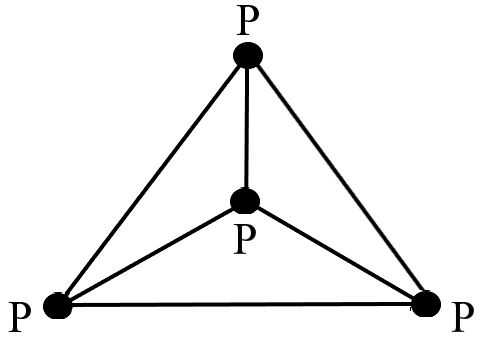
Several approaches can be used to produce the white allotrope; the most common one is shown below.
Phosphate rock (containing fluorapatite $C{a_5}{(P{O_4})_3}F$) on heating when placed within a fuel-fired/electric furnace along with traces of silica and carbon; is one such industrial process we can use to obtain white phosphorous. This is because the element phosphorus turns into a vapor form and starts to disperse or liberate. The white phosphorous can then be obtained by collecting the vapors in phosphoric acid.
If we need to write a chemical equation for the same, we can replace phosphate rock with calcium phosphate and create the chemical equation:
$2C{a_3}{(P{O_4})_2} + 6Si{O_2} + 10C \to 6CaSi{O_3} + 10CO + {P_4}$
Note:
White phosphorus is a white waxy material with a transparent appearance that is able to glow when placed in the dark. Red phosphorus can be produced after several days of heating white phosphorus at 573K in an inert environment. Furthermore, when heated at high pressure, it forms a sequence of black forms.
Complete answer:
In nature, there are various allotropic sources of phosphorus.
The various forms result from the various ways atoms can be bound together. White phosphorus is one such allotrope that exists for phosphorus.
The white phosphorus allotrope would be a waxy solid that explodes or burns readily and is used in chemical processing or in smoke explosives.
White phosphorus (yellow phosphorus) also known as tetraphosphorus (${P_4}$) occur as tetrahedral molecules that are comprised of four atoms of the same kind. The molecule is said to be made up of six independent single P–P bonds.
The figure showing the structure of a white phosphorous is illustrated below:

Several approaches can be used to produce the white allotrope; the most common one is shown below.
Phosphate rock (containing fluorapatite $C{a_5}{(P{O_4})_3}F$) on heating when placed within a fuel-fired/electric furnace along with traces of silica and carbon; is one such industrial process we can use to obtain white phosphorous. This is because the element phosphorus turns into a vapor form and starts to disperse or liberate. The white phosphorous can then be obtained by collecting the vapors in phosphoric acid.
If we need to write a chemical equation for the same, we can replace phosphate rock with calcium phosphate and create the chemical equation:
$2C{a_3}{(P{O_4})_2} + 6Si{O_2} + 10C \to 6CaSi{O_3} + 10CO + {P_4}$
Note:
White phosphorus is a white waxy material with a transparent appearance that is able to glow when placed in the dark. Red phosphorus can be produced after several days of heating white phosphorus at 573K in an inert environment. Furthermore, when heated at high pressure, it forms a sequence of black forms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




