
Predict the product of the following reaction:
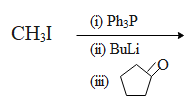
A. $\text{C}{{\text{H}}_{\text{2}}}\text{CHO}$
B.
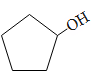
C.
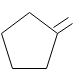
D.
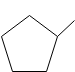
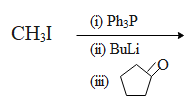



Answer
601.8k+ views
Hint: The name of the reaction which is mentioned in the question is Wittig reaction. We can determine the product accordingly.
Complete step-by-step answer:
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide. It is widely used in organic synthesis for the preparation of alkenes.
The procedure in the Wittig reaction can be divided into two arep. In the first step, a phosphorus ylide is prepared by treating a suitable phosphonium salt with a base. In the second step the ylide is reacted with a substrate containing a carbonyl group to give the desired alkene.
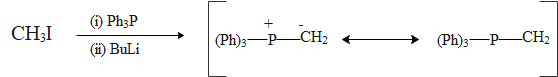
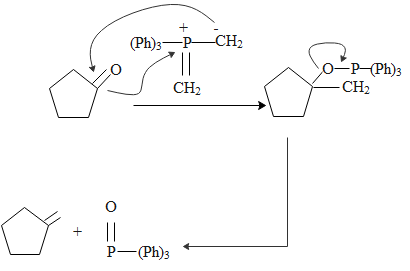
As we can see that the product of the reaction that we can obtain is :
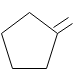
So the correct answer to the question is Option C.
Additional Information:
Occasionally it is also stated that, Wittig reaction is a reversible reaction. This statement is based on the procedure to provide carbonyl compound and phosphorane as a possible product. Such a reaction is known as the retro- Wittig reaction.
The most important uses of ylides in synthesis comes from their reactions with aldehydes and ketones, which are initiated in every case. Covalent bonding of the nucleophilic alpha carbon to the electrophilic carbonyl carbon.
Note: The formation of a phosphine oxide with a strong phosphorus and oxygen bond is the driving force for the classical Wittig reaction, but is wasteful and can put problems during purification. The limiting reagent in the Wittig reaction is triphenylphosphine.
Complete step-by-step answer:
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide. It is widely used in organic synthesis for the preparation of alkenes.
The procedure in the Wittig reaction can be divided into two arep. In the first step, a phosphorus ylide is prepared by treating a suitable phosphonium salt with a base. In the second step the ylide is reacted with a substrate containing a carbonyl group to give the desired alkene.
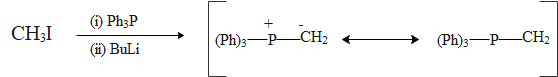

As we can see that the product of the reaction that we can obtain is :

So the correct answer to the question is Option C.
Additional Information:
Occasionally it is also stated that, Wittig reaction is a reversible reaction. This statement is based on the procedure to provide carbonyl compound and phosphorane as a possible product. Such a reaction is known as the retro- Wittig reaction.
The most important uses of ylides in synthesis comes from their reactions with aldehydes and ketones, which are initiated in every case. Covalent bonding of the nucleophilic alpha carbon to the electrophilic carbonyl carbon.
Note: The formation of a phosphine oxide with a strong phosphorus and oxygen bond is the driving force for the classical Wittig reaction, but is wasteful and can put problems during purification. The limiting reagent in the Wittig reaction is triphenylphosphine.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




