
${\text{Phthalic acid}} + {\text{N}}{{\text{H}}_3} \to {\text{D}}\xrightarrow{{{\Delta }}}{\text{E}}$
Answer
522.3k+ views
Hint:To solve this we must know the structure of phthalic acid. Phthalic acid in reaction with ammonia gives an ammonium salt. The ammonium salt on heating gives phthalimide. Write the reaction along with the proper structures.
Complete step by step answer:We are given that phthalic acid reacts with ammonia.
Phthalic acid is a carboxylic acid. The structure of phthalic acid has two carboxyl groups attached to the benzene ring.
Phthalic acid is a weak acid and ammonia is a weak base. Thus, ammonia accepts a proton and forms ammonium phthalate.
Phthalic acid has a carboxyl group and ammonia is a base. The acid donates a hydrogen ion and the ammonia molecule accepts hydrogen ions. This produces ammonium salt of phthalic acid. The salt is known as ammonium phthalate.
The reaction of phthalic acid with ammonia is as follows:
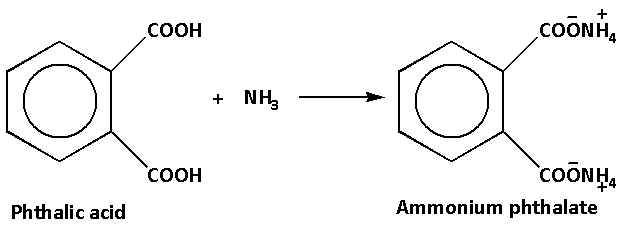
Ammonium phthalate on heating further loses two water molecules. When ammonium phthalate loses water molecules phthalimide is produced. Phthalimide is an imide derivative of phthalic anhydride.
The reaction when ammonium phthalate is heated is as follows:
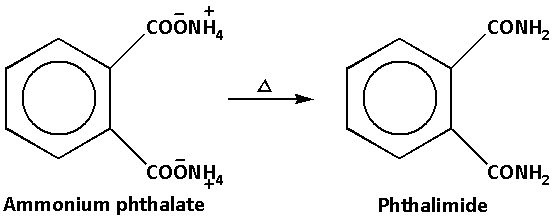
Thus, product D is ammonium phthalate and product E is phthalimide.
Thus, ${\text{Phthalic acid}} + {\text{N}}{{\text{H}}_3} \to {\text{Ammonium phthalate}}\xrightarrow{{{\Delta }}}{\text{Phthalimide}}$.
Note: Phthalic acid is weak acid and ammonia is a weak base. The acid reacts with base and forms salt and water. The weak phthalic acid donates a proton to ammonia which is a weak base. The weak base ammonia accepts a proton to form ammonium phthalate. Further heating of ammonium phthalate leads to loss of water and leads to the formation of phthalimide.
Complete step by step answer:We are given that phthalic acid reacts with ammonia.
Phthalic acid is a carboxylic acid. The structure of phthalic acid has two carboxyl groups attached to the benzene ring.
Phthalic acid is a weak acid and ammonia is a weak base. Thus, ammonia accepts a proton and forms ammonium phthalate.
Phthalic acid has a carboxyl group and ammonia is a base. The acid donates a hydrogen ion and the ammonia molecule accepts hydrogen ions. This produces ammonium salt of phthalic acid. The salt is known as ammonium phthalate.
The reaction of phthalic acid with ammonia is as follows:

Ammonium phthalate on heating further loses two water molecules. When ammonium phthalate loses water molecules phthalimide is produced. Phthalimide is an imide derivative of phthalic anhydride.
The reaction when ammonium phthalate is heated is as follows:

Thus, product D is ammonium phthalate and product E is phthalimide.
Thus, ${\text{Phthalic acid}} + {\text{N}}{{\text{H}}_3} \to {\text{Ammonium phthalate}}\xrightarrow{{{\Delta }}}{\text{Phthalimide}}$.
Note: Phthalic acid is weak acid and ammonia is a weak base. The acid reacts with base and forms salt and water. The weak phthalic acid donates a proton to ammonia which is a weak base. The weak base ammonia accepts a proton to form ammonium phthalate. Further heating of ammonium phthalate leads to loss of water and leads to the formation of phthalimide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




