
What is the phosphodiester bond and how it is drawn?
Answer
564.6k+ views
Hint:To solve this question, first we have to understand the term phosphodiester bond. To draw a phosphodiester bond, we should have prior knowledge about the connectivity which leads to the formation of the bond.
Complete answer:
Phosphodiester bond is universal to all the life forms which are present on the earth because the phosphodiester bond is present in the backbone of both DNA and RNA. DNA stands for deoxy ribonucleic acid and RNA stands for ribonucleic acid. The DNA consists of two polynucleotide chains which are connected by the strong electromagnetic attraction also known as the phosphodiester bond.
The Phosphodiester bond is a covalent bond which is generally formed between the phosphate group of ${{5}^{'}}$ carbon of one of the nucleotides and ${{3}^{'}}$ carbon of another nucleotide through the formation of ester linkage. This bond is formed due to the condensation reaction occurring between the hydroxyl group of two sugar groups and the one phosphate group and a bond which is responsible for the linkage of ${{5}^{'}}$ carbon of one of the nucleotides and ${{3}^{'}}$ carbon of another nucleotide through the formation of ester linkage in DNA and RNA.
Phosphodiester linkage is shown below:
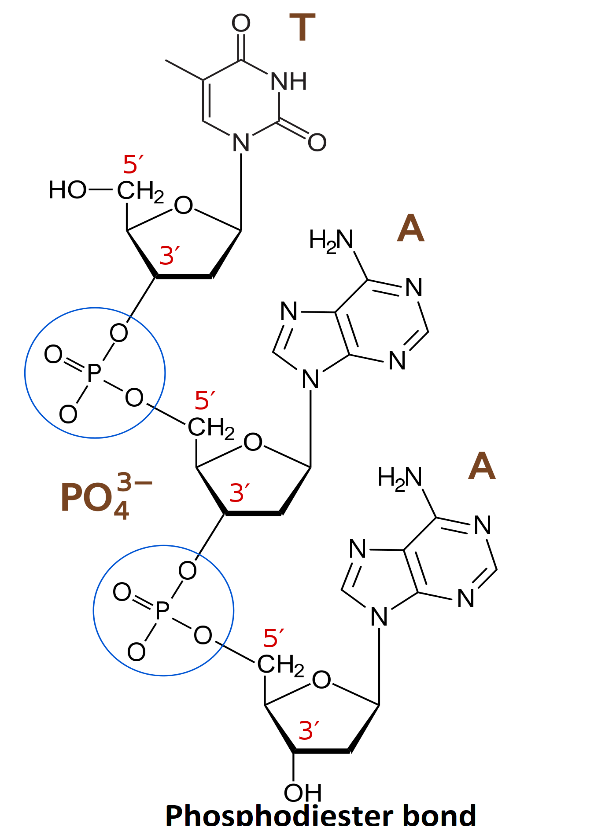
Note:
The formation of Phosphodiester bonds is a synthetic process or also known as building up process. Thus, it is an anabolic process. An anabolic process is the process in which energy is used along with the elimination of the water molecule. The bond formed in the Phosphodiester bond is a covalent bond.
Complete answer:
Phosphodiester bond is universal to all the life forms which are present on the earth because the phosphodiester bond is present in the backbone of both DNA and RNA. DNA stands for deoxy ribonucleic acid and RNA stands for ribonucleic acid. The DNA consists of two polynucleotide chains which are connected by the strong electromagnetic attraction also known as the phosphodiester bond.
The Phosphodiester bond is a covalent bond which is generally formed between the phosphate group of ${{5}^{'}}$ carbon of one of the nucleotides and ${{3}^{'}}$ carbon of another nucleotide through the formation of ester linkage. This bond is formed due to the condensation reaction occurring between the hydroxyl group of two sugar groups and the one phosphate group and a bond which is responsible for the linkage of ${{5}^{'}}$ carbon of one of the nucleotides and ${{3}^{'}}$ carbon of another nucleotide through the formation of ester linkage in DNA and RNA.
Phosphodiester linkage is shown below:

Note:
The formation of Phosphodiester bonds is a synthetic process or also known as building up process. Thus, it is an anabolic process. An anabolic process is the process in which energy is used along with the elimination of the water molecule. The bond formed in the Phosphodiester bond is a covalent bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




