
Phenylacetylene on treatment with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ produces:
A. Acetophenone
B. Phenylacetaldehyde
C. Phenylacetic acid
D. 1-Phenylethanol
E. 2-Phenylethanol
Answer
580.2k+ views
Hint: Generally alkenes reacts with strong acids and forms respective alcohols or ketones as the product. But alkynes won’t react with strong acids, alkynes undergoes hydration reaction in the presence of mercuric sulphate catalyst and forms the respective ketone or aldehyde as the product.
Complete answer:
- In the question it is given that the treatment of phenylacetylene with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ produces which product.
- Reaction of alkynes with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ is an example for hydration reaction on alkynes using mercuric sulphate as the reagent.
- The reaction of phenylacetylene with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ is as follows.
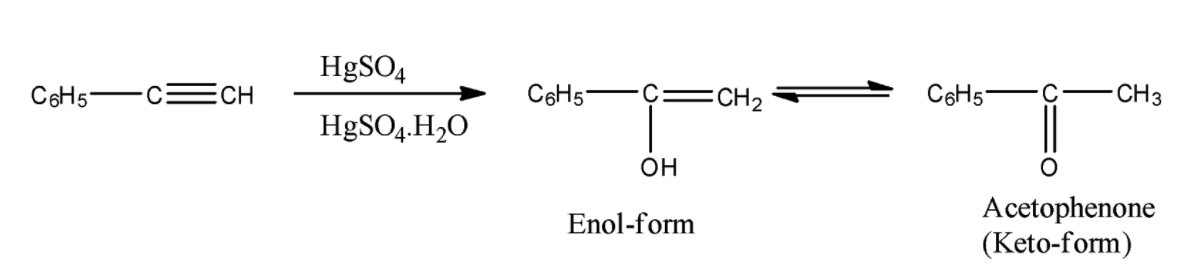
- In the above reaction phenylacetylene reacts with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ and forms an enol as the product.
- We know that enol form is unstable at room temperature and converts to keto-form and the product name is acetophenone.
- Therefore the reaction of Phenylacetylene with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ produces acetophenone as the product.
- So, the correct option is A.
Additional information:
- The reaction of alkynes with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ and produces a aldehyde or ketone as the product is called Kucherov’s reaction.
- This is a type of hydration reaction and it is not possible by using strong acids as the reagent.
- Strong acids can react with alkenes and form respective products.
Note: If the alkynes are highly substituted then the alkynes gives ketone as the product on reaction with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$. If the alkynes are less substituted then the less substituted alkynes gives aldehyde as the product.
Complete answer:
- In the question it is given that the treatment of phenylacetylene with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ produces which product.
- Reaction of alkynes with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ is an example for hydration reaction on alkynes using mercuric sulphate as the reagent.
- The reaction of phenylacetylene with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ is as follows.

- In the above reaction phenylacetylene reacts with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ and forms an enol as the product.
- We know that enol form is unstable at room temperature and converts to keto-form and the product name is acetophenone.
- Therefore the reaction of Phenylacetylene with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ produces acetophenone as the product.
- So, the correct option is A.
Additional information:
- The reaction of alkynes with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$ and produces a aldehyde or ketone as the product is called Kucherov’s reaction.
- This is a type of hydration reaction and it is not possible by using strong acids as the reagent.
- Strong acids can react with alkenes and form respective products.
Note: If the alkynes are highly substituted then the alkynes gives ketone as the product on reaction with $HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}.{{H}_{2}}O$. If the alkynes are less substituted then the less substituted alkynes gives aldehyde as the product.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




