
Phenols show less acidity in comparison to carboxylic acid. Explain
Answer
522.3k+ views
Hint: We also remember that the general formula of alcohol is \[{C_n}{H_{2n + 1}}OH\]. Alcohol and phenol have one thing in common that they both have hydroxyl groups present in it. Alcohol is an aliphatic functional group whereas phenol is an aromatic functional group. Carboxylic acids have \[-COOH\] functional groups.
Complete answer:
We also know that the phenol is an organic compounds containing the hydroxyl group i.e. \[ - OH\]with a benzene ring.
Phenol is an aromatic organic compound that contains hydroxyl group which is attached to a benzene ring therefore the functional group is \[-{C_6}{H_5}OH\] or
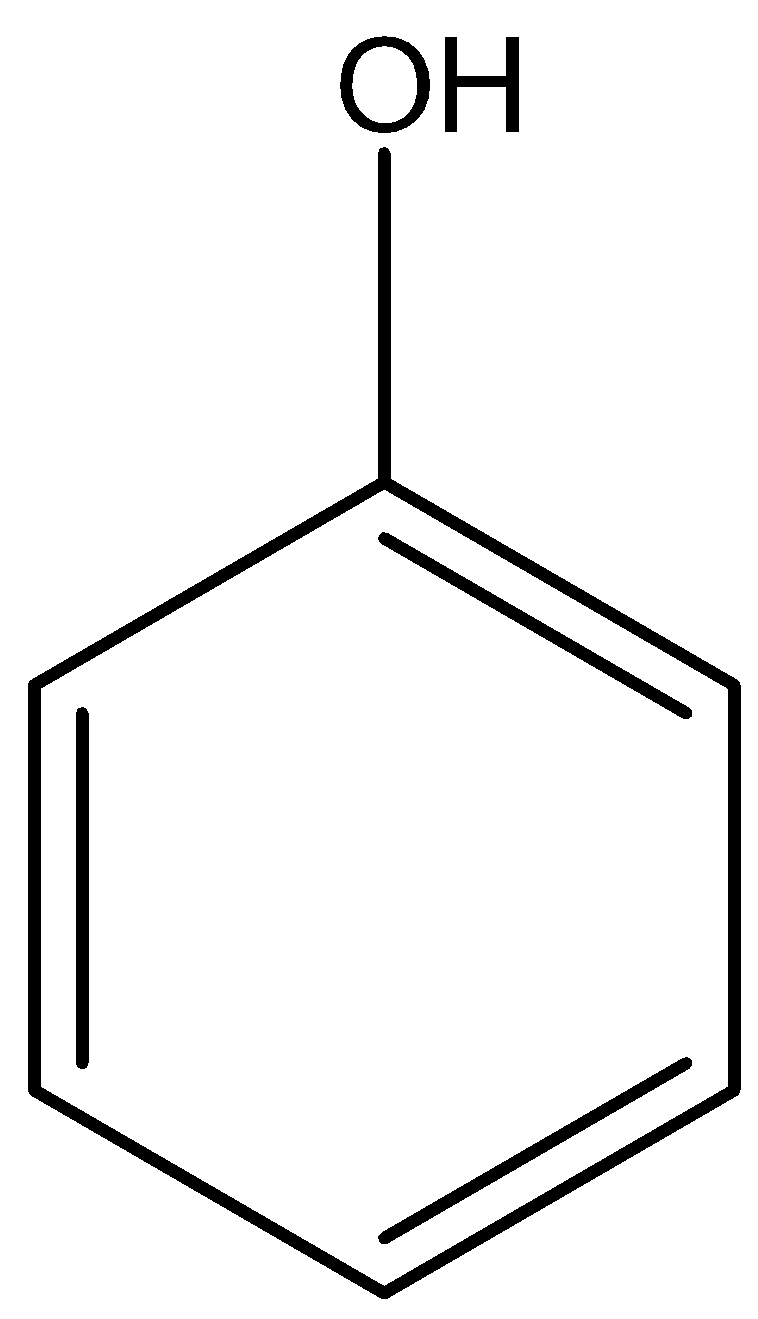
Phenol is a weak acid that has a pH of $8 - 12$. There is resonance stabilization in phenol due to which it is more acidic than aliphatic alcohols.
Carboxylic acid is an organic compound having a functional group \[-COOH\].
Phenols are weaker acids than carboxylic acids even though there is delocalization of charge over the aromatic ring of the phenoxide ion. The carboxylate ion is more stabilized relative to the phenoxide ion because the negative charge is located on the oxygen atoms of the carboxylate ion.
When phenol and carboxylic acid dissociates itself into ions they form phenoxide ion and carboxylate ion that can be represented below:
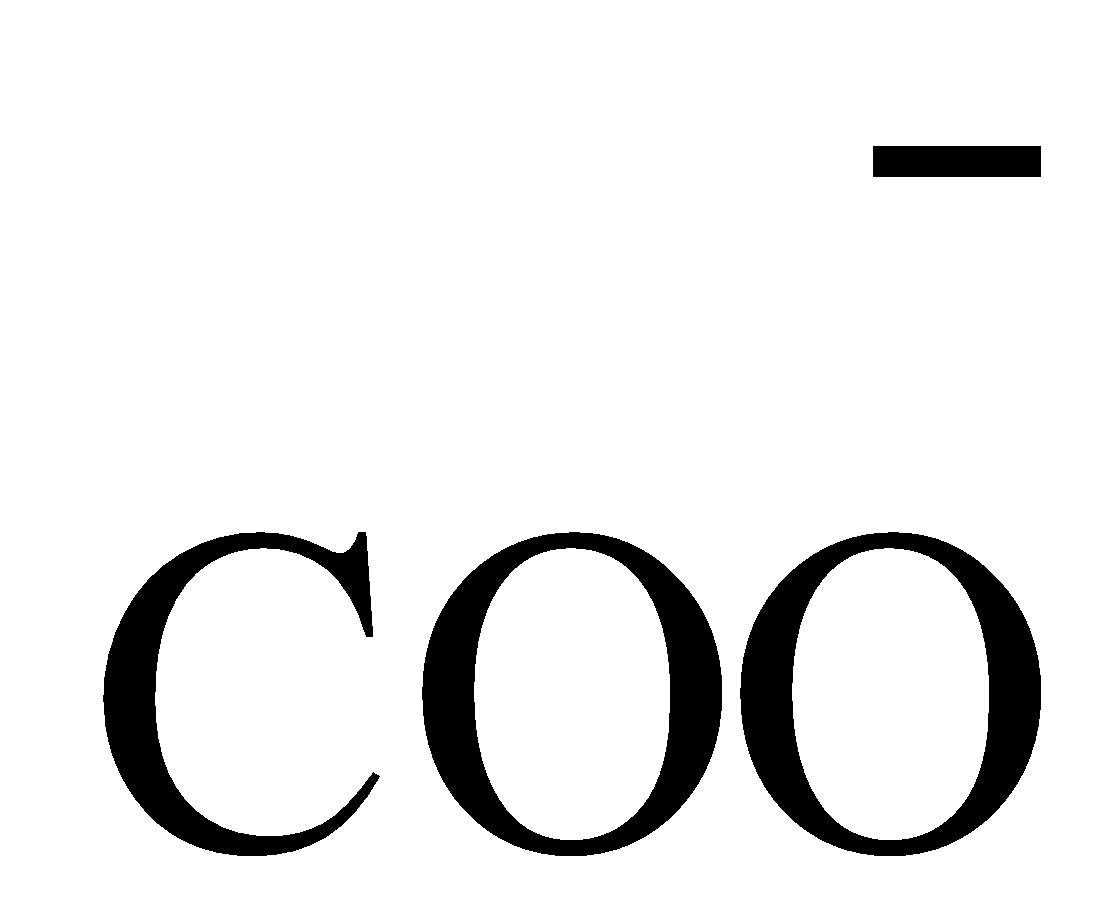
Carboxylate ion
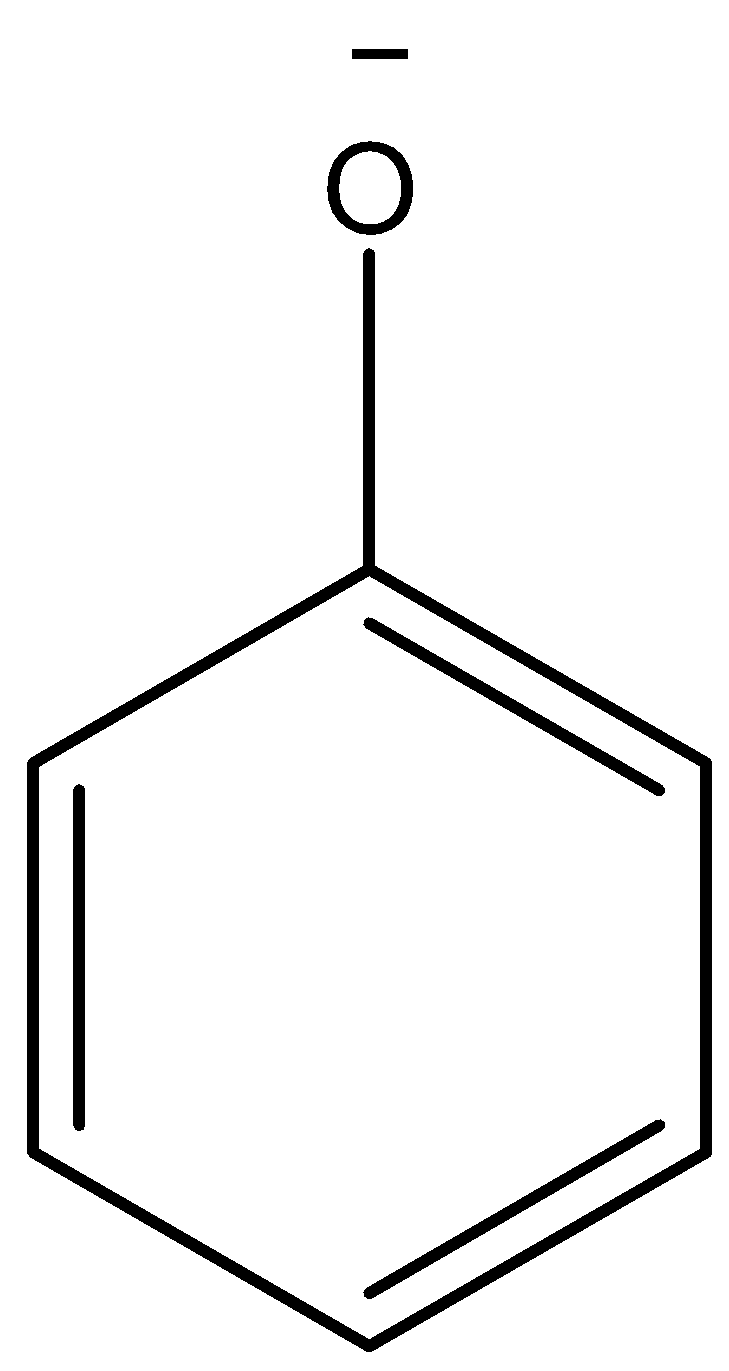 phenolate ion
phenolate ion
Note:
We have to know that in case of phenols, negative charge is less effectively delocalized over one oxygen atom and less electronegative carbon atoms in phenoxide ions. Therefore, the carboxylate ion exhibits higher stability in comparison to phenoxide ions. Hence, the carboxylic acids are more acidic than phenols. Phenols have generally higher acidity than alcohols.
Complete answer:
We also know that the phenol is an organic compounds containing the hydroxyl group i.e. \[ - OH\]with a benzene ring.
Phenol is an aromatic organic compound that contains hydroxyl group which is attached to a benzene ring therefore the functional group is \[-{C_6}{H_5}OH\] or

Phenol is a weak acid that has a pH of $8 - 12$. There is resonance stabilization in phenol due to which it is more acidic than aliphatic alcohols.
Carboxylic acid is an organic compound having a functional group \[-COOH\].
Phenols are weaker acids than carboxylic acids even though there is delocalization of charge over the aromatic ring of the phenoxide ion. The carboxylate ion is more stabilized relative to the phenoxide ion because the negative charge is located on the oxygen atoms of the carboxylate ion.
When phenol and carboxylic acid dissociates itself into ions they form phenoxide ion and carboxylate ion that can be represented below:
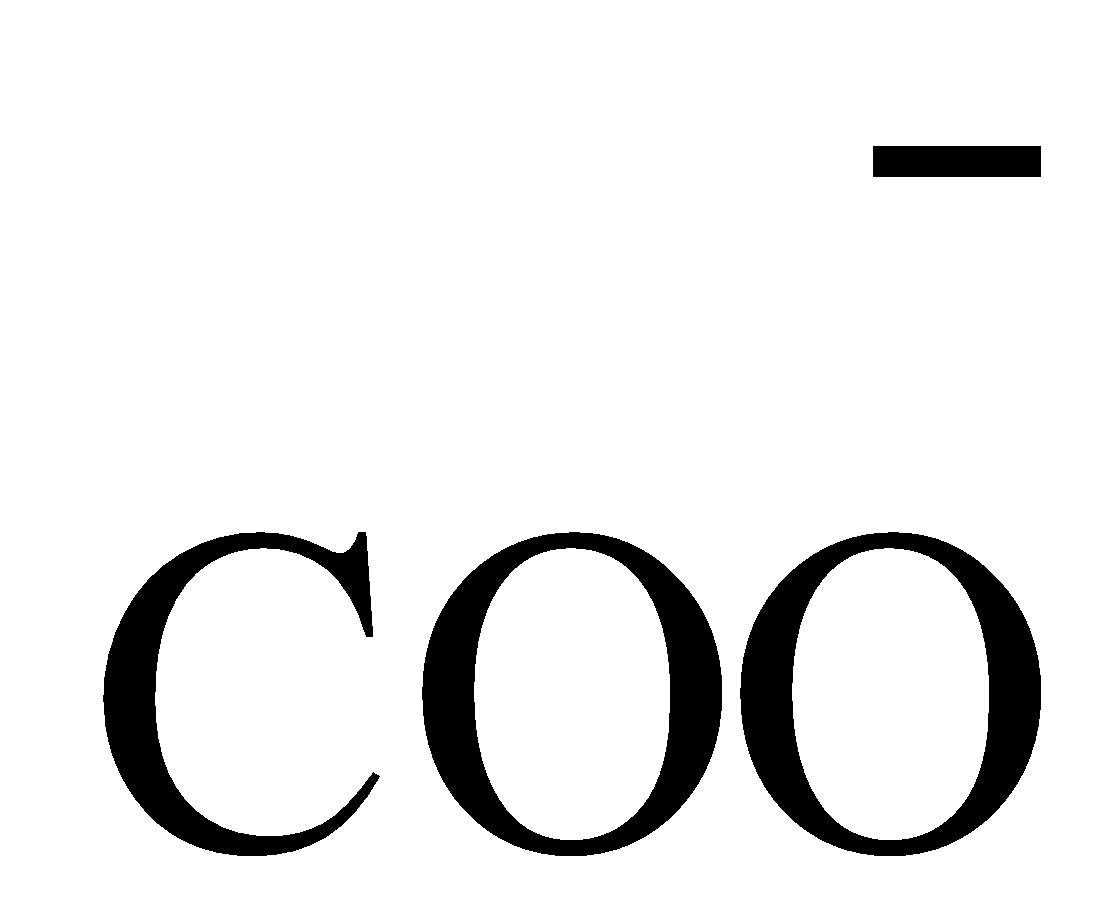
Carboxylate ion

Note:
We have to know that in case of phenols, negative charge is less effectively delocalized over one oxygen atom and less electronegative carbon atoms in phenoxide ions. Therefore, the carboxylate ion exhibits higher stability in comparison to phenoxide ions. Hence, the carboxylic acids are more acidic than phenols. Phenols have generally higher acidity than alcohols.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction




