
When phenolphthalein is added to$NaOH$, the colour of the solution will become _________
(a)- Colorless
(b)- Red
(c)- Pink
(d)- Yellow
Answer
590.4k+ views
Hint: In a neutral solution, the phenolphthalein would give a light pink colour. When phenolphthalein is added to the base the colour of the solution intensifies and if it is added to an acidic solution the colour will fade away and become colourless.
Complete step by step answer:
Phenolphthalein is a chemical compound that is used as an indicator in acid-base titrations.
The chemical formula of phenolphthalein is${{C}_{20}}{{H}_{14}}{{O}_{4}}$.
Structure of phenolphthalein is given below:
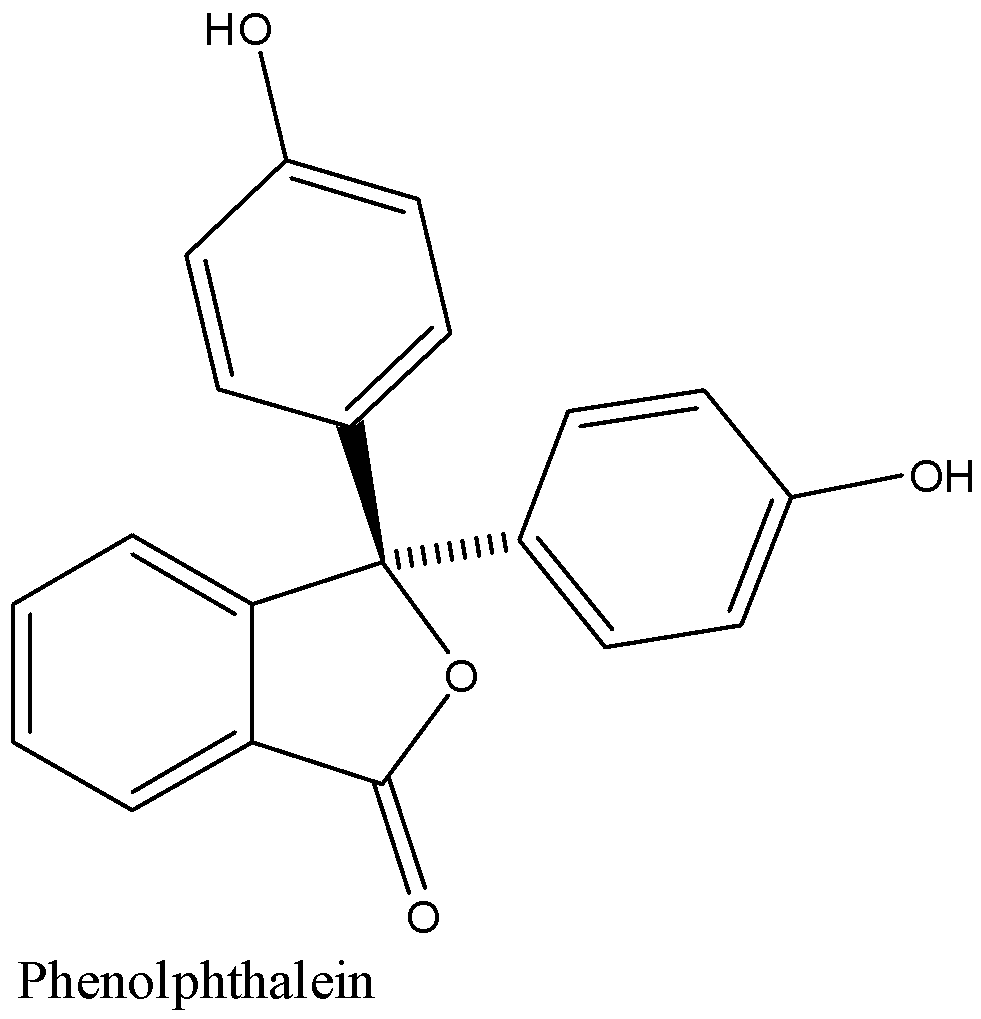
In acidic medium the colour of phenolphthalein is colourless and in the basic medium, it has a very dark pink colour.
The reaction with acid and base is given below:
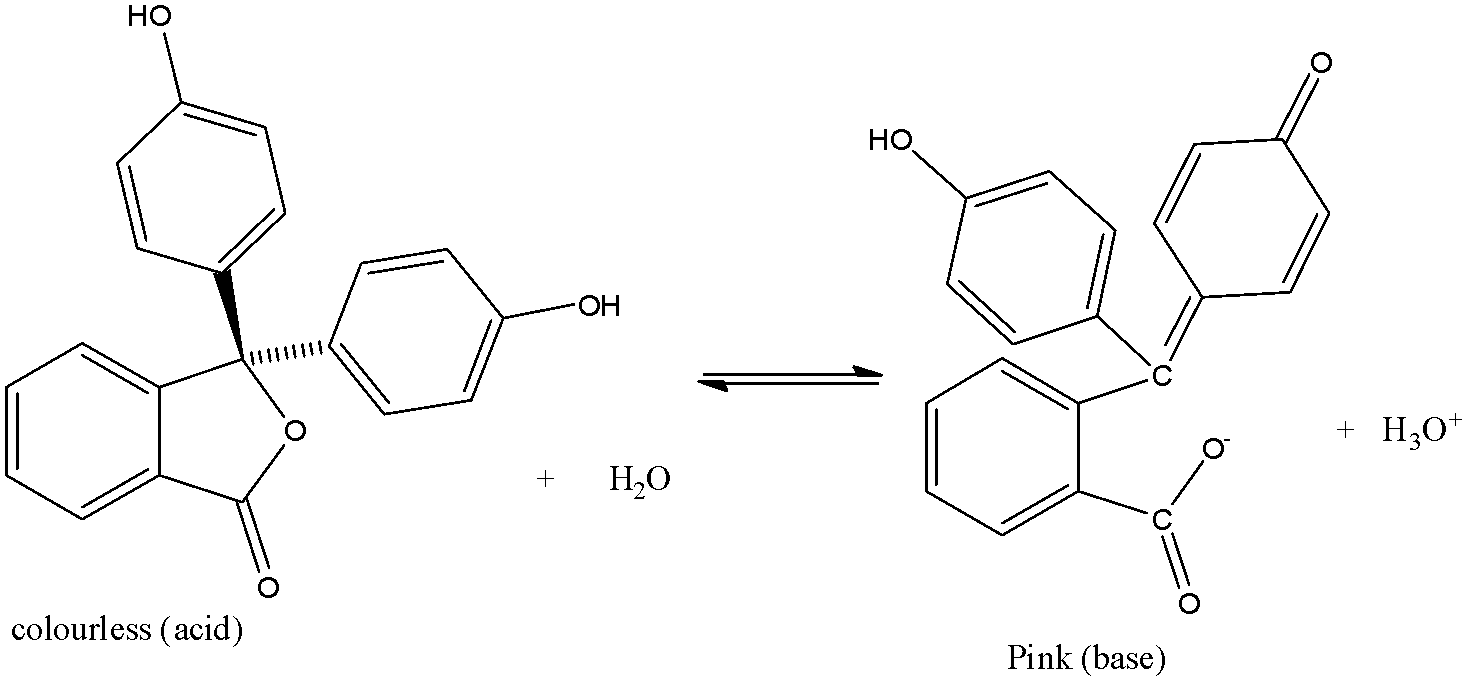
It mostly dissolves in alcohol and is slightly soluble in water.
When dissolved in water it can lose ${{H}^{+}}$ions and form phenolphthalein ions. The phenolphthalein ion is pink and the phenolphthalein molecule is colourless.
When the phenolphthalein is dissolved in the base the ionization starts and the formation of phenolphthalein ion increases, this makes the solution dark pink.
But when the acid is added, the phenolphthalein ion gets converted into phenolphthalein molecule making the solution colourless.
Earlier phenolphthalein was used as a laxative in the medical field. A laxative is used for the treatment of constipation. But it is being removed as a laxative because it is carcinogenic. It restricts the function of calcium ions in the body.
So, $NaOH$is a base, and the phenolphthalein gives pink colour in the basic solution.
Hence, the correct answer is an option (c)- Pink.
Note: If the phenolphthalein is added to a very acidic solution then it would give an orange colour. When phenolphthalein is added to a highly basic solution then the colour changes from pink to colourless because it forms$\ln {{(OH)}^{3-}}$ ions. It is used for the study of reaction kinetics.
Complete step by step answer:
Phenolphthalein is a chemical compound that is used as an indicator in acid-base titrations.
The chemical formula of phenolphthalein is${{C}_{20}}{{H}_{14}}{{O}_{4}}$.
Structure of phenolphthalein is given below:

In acidic medium the colour of phenolphthalein is colourless and in the basic medium, it has a very dark pink colour.
The reaction with acid and base is given below:

It mostly dissolves in alcohol and is slightly soluble in water.
When dissolved in water it can lose ${{H}^{+}}$ions and form phenolphthalein ions. The phenolphthalein ion is pink and the phenolphthalein molecule is colourless.
When the phenolphthalein is dissolved in the base the ionization starts and the formation of phenolphthalein ion increases, this makes the solution dark pink.
But when the acid is added, the phenolphthalein ion gets converted into phenolphthalein molecule making the solution colourless.
Earlier phenolphthalein was used as a laxative in the medical field. A laxative is used for the treatment of constipation. But it is being removed as a laxative because it is carcinogenic. It restricts the function of calcium ions in the body.
So, $NaOH$is a base, and the phenolphthalein gives pink colour in the basic solution.
Hence, the correct answer is an option (c)- Pink.
Note: If the phenolphthalein is added to a very acidic solution then it would give an orange colour. When phenolphthalein is added to a highly basic solution then the colour changes from pink to colourless because it forms$\ln {{(OH)}^{3-}}$ ions. It is used for the study of reaction kinetics.
Recently Updated Pages
Master Class 7 English: Engaging Questions & Answers for Success

Master Class 7 Maths: Engaging Questions & Answers for Success

Master Class 7 Science: Engaging Questions & Answers for Success

Class 7 Question and Answer - Your Ultimate Solutions Guide

Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Trending doubts
The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

Convert 200 Million dollars in rupees class 7 maths CBSE

List of coprime numbers from 1 to 100 class 7 maths CBSE

AIM To prepare stained temporary mount of onion peel class 7 biology CBSE

The plural of Chief is Chieves A True B False class 7 english CBSE

Write a letter to the editor of the national daily class 7 english CBSE





