
Phenol and benzoic acid can be distinguished by:
a. Aqueous\[{{N}_{a}}HC{{O}_{3}}\]
b. Aqueous\[{{N}_{a}}N{{O}_{3}}\]
c. Aqueous\[{{N}_{a}}OH\]
d. Conc. \[{{H}_{2}}S{{O}_{4}}\]
Answer
570k+ views
Hint: Benzoic acid and phenol, both are acidic in nature. However benzoic acid is a stronger acid than phenol. This property of acid strength can help in distinguishing phenol and benzoic acid.
Complete Step by step answer: Four options one of which helps in distinguishing phenol and benzoic acid.
Before answering the question let us first understand the acid strength of benzoic acid and phenol which will further enable us to answer the given question. The conjugate base of benzoic acid is benzoate ion which is stabilized by two equivalent resonance structures in which the negative charge is present at the more electronegative oxygen atom.
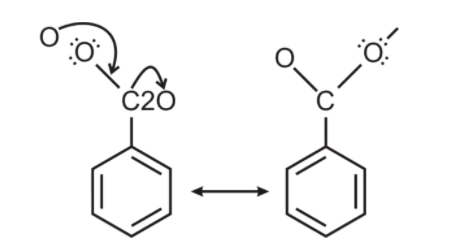
However the conjugate base of phenol, a phenoxide ion has non- equivalent resonance structure in which the negative charge is at the less electronegative carbon atom.
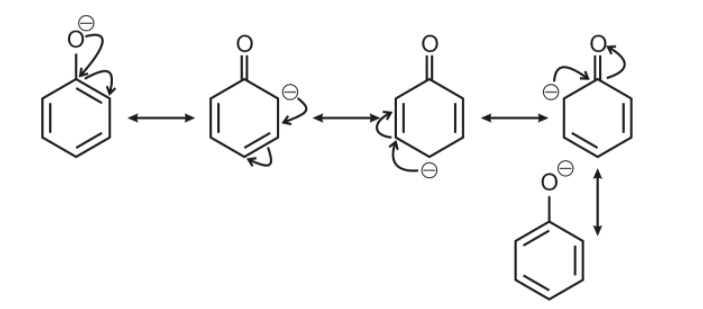
Thus the benzoate ion is more stable than phenoxide ion. Hence, benzoic acid is a stronger acid than phenol. Hence phenol, being very weak acids do not react with weak bases such as sodium hydrogen carbonate.
However, benzoic acid being a strong acid, reacts with sodium hydrogen carbonate to give effervescence of carbon dioxide gas.
\[{{C}_{6}}{{H}_{5}}OH+{{N}_{a}}HC{{O}_{3}}\to \] No Reaction
(Phenol)
\[{{C}_{6}}{{H}_{5}}-COOH+{{N}_{a}}HC{{O}_{3}}\to {{C}_{6}}{{H}_{5}}-COONa+{{H}_{2}}O+C{{o}_{2}}\]
(Benzoic acid)
Thus option (A) is the correct option.
Note: Students should note that there are many other dry and out tests to distinguish between phenol and benzoic acid, one with freshly prepared ferric chloride solutions gives distinctive colours. Phenol gives violet color due to formation of iron- phenol complex white benzoic acid gives a buff colour due to formation of ferric benzoate.
Complete Step by step answer: Four options one of which helps in distinguishing phenol and benzoic acid.
Before answering the question let us first understand the acid strength of benzoic acid and phenol which will further enable us to answer the given question. The conjugate base of benzoic acid is benzoate ion which is stabilized by two equivalent resonance structures in which the negative charge is present at the more electronegative oxygen atom.

However the conjugate base of phenol, a phenoxide ion has non- equivalent resonance structure in which the negative charge is at the less electronegative carbon atom.

Thus the benzoate ion is more stable than phenoxide ion. Hence, benzoic acid is a stronger acid than phenol. Hence phenol, being very weak acids do not react with weak bases such as sodium hydrogen carbonate.
However, benzoic acid being a strong acid, reacts with sodium hydrogen carbonate to give effervescence of carbon dioxide gas.
\[{{C}_{6}}{{H}_{5}}OH+{{N}_{a}}HC{{O}_{3}}\to \] No Reaction
(Phenol)
\[{{C}_{6}}{{H}_{5}}-COOH+{{N}_{a}}HC{{O}_{3}}\to {{C}_{6}}{{H}_{5}}-COONa+{{H}_{2}}O+C{{o}_{2}}\]
(Benzoic acid)
Thus option (A) is the correct option.
Note: Students should note that there are many other dry and out tests to distinguish between phenol and benzoic acid, one with freshly prepared ferric chloride solutions gives distinctive colours. Phenol gives violet color due to formation of iron- phenol complex white benzoic acid gives a buff colour due to formation of ferric benzoate.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




