
Peroxy-disulphuric acid (\[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]) can be prepared by electrolytic oxidation of \[{{H}_{2}}S{{O}_{4}}\] as:
\[2{{H}_{2}}S{{O}_{4}}\to {{H}_{2}}{{S}_{2}}{{O}_{8}}+2{{H}^{+}}+2{{e}^{-}}\]
Oxygen and hydrogen are by-products. In such an electrolysis 9.72 litre of \[{{H}_{2}}\] and 2.35 litre of \[{{O}_{2}}\] were generated at NTP. What is the mass of peroxy-disulphuric acid formed?
Answer
601.5k+ views
Hint: Remember that one mole of gas occupies 22.4 litres of volume at NTP. Also remember that any electrochemical reactions can be divided into two half-reactions: cathodic and anodic half-reactions.
Step-by-Step Solution:
Let us first look into peroxy disulphuric acid as a compound before moving onto the solution of this question.
Peroxy disulfuric acid is the inorganic compound with the chemical formula \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]. Also called Marshall's acid after its inventor Professor Hugh Marshall, it is a sulphur oxoacid. It contains sulphur in its +6-oxidation state and a peroxide group. Its salts, commonly known as persulfates, are industrially important as powerful oxidizing agents.
Let us now move onto the solution of this question,
Observing the anode reaction at the very beginning:
\[2{{H}_{2}}S{{O}_{4}}\to {{H}_{2}}{{S}_{2}}{{O}_{8}}+2{{H}^{+}}+2{{e}^{-}}\]
And
$2{{H}_{2}}O\to 4{{H}^{+}}+{{O}_{2}}+2{{e}^{-}}$
Now observing the cathode half reaction:
\[2{{H}_{2}}O+2{{e}^{-}}\to 2O{{H}^{-}}+{{H}_{2}}\]
Now, upon balancing the equivalents of the products of the cathode and anode half reactions, we get:
Equivalents of \[{{O}_{2}}\] + Equivalents of \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\] = Equivalents of \[{{H}_{2}}\]
Now, given the volumes of Oxygen and Hydrogen let us now calculate the number of their equivalents formed respectively.
Equivalents of \[{{H}_{2}}\]= $\dfrac{9.72}{11.2}=0.868$equivalents
Equivalents of \[{{O}_{2}}\]= $\dfrac{2.35}{5.6}=0.42$equivalents
Now, plugging these equivalent values into the balanced equation of cathode and anode half reactions.
Therefore, Equivalents of \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]= 0.868 – 0.42 = 0.448
Mass of \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]= $0.448\times \dfrac{194}{2}=43.46g$
Thus, we can conclude that the mass of peroxy disulphuric acid here is 43.46g
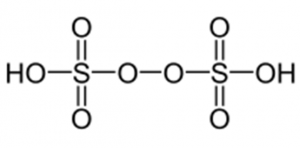
Note: Peroxy disulphuric acid contains one peroxide group forming a bridge between the two sulphur atoms. Each sulphur atom is connected to one hydroxyl group (S-OH bond) and two oxygen atoms (S=O bond) other than the peroxide group.
Step-by-Step Solution:
Let us first look into peroxy disulphuric acid as a compound before moving onto the solution of this question.
Peroxy disulfuric acid is the inorganic compound with the chemical formula \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]. Also called Marshall's acid after its inventor Professor Hugh Marshall, it is a sulphur oxoacid. It contains sulphur in its +6-oxidation state and a peroxide group. Its salts, commonly known as persulfates, are industrially important as powerful oxidizing agents.
Let us now move onto the solution of this question,
Observing the anode reaction at the very beginning:
\[2{{H}_{2}}S{{O}_{4}}\to {{H}_{2}}{{S}_{2}}{{O}_{8}}+2{{H}^{+}}+2{{e}^{-}}\]
And
$2{{H}_{2}}O\to 4{{H}^{+}}+{{O}_{2}}+2{{e}^{-}}$
Now observing the cathode half reaction:
\[2{{H}_{2}}O+2{{e}^{-}}\to 2O{{H}^{-}}+{{H}_{2}}\]
Now, upon balancing the equivalents of the products of the cathode and anode half reactions, we get:
Equivalents of \[{{O}_{2}}\] + Equivalents of \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\] = Equivalents of \[{{H}_{2}}\]
Now, given the volumes of Oxygen and Hydrogen let us now calculate the number of their equivalents formed respectively.
Equivalents of \[{{H}_{2}}\]= $\dfrac{9.72}{11.2}=0.868$equivalents
Equivalents of \[{{O}_{2}}\]= $\dfrac{2.35}{5.6}=0.42$equivalents
Now, plugging these equivalent values into the balanced equation of cathode and anode half reactions.
Therefore, Equivalents of \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]= 0.868 – 0.42 = 0.448
Mass of \[{{H}_{2}}{{S}_{2}}{{O}_{8}}\]= $0.448\times \dfrac{194}{2}=43.46g$
Thus, we can conclude that the mass of peroxy disulphuric acid here is 43.46g
Note: Peroxy disulphuric acid contains one peroxide group forming a bridge between the two sulphur atoms. Each sulphur atom is connected to one hydroxyl group (S-OH bond) and two oxygen atoms (S=O bond) other than the peroxide group.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




