
Pentan-2-one is obtained from
(A) 2,2-Dichloropentane
(B)3,3-dichloropentane
(C)Pentan-3-ol
(D)Pent-2-yne
Answer
574.8k+ views
Hint: Methyl propyl ketone which is the other name for Pentan-2-one, can be prepared from the reactant by undergoing hydration followed by dehydration reaction. Hydration reaction involves the addition of water molecules and dehydration involves the removal of water molecules.
Complete step by step answer:
Pentan-2-one is also known as methyl propyl ketone. Pent-2-one can be prepared from 2,2-Dichloropentane. In this reaction, the reactant contains two chlorine atoms. The reactant will undergo hydrolysis to give Pentane-2,2-diol. This happens when the chlorine atoms present in 2,2-Dichloropentane are replaced by the -OH group in order to give Pentane-2,2-diol. Hydration is a process which involves the addition of the water molecule.
Further the formed pentane-2,2-diol will undergo dehydration reaction. Dehydration is a process that involves removal of the water molecule. It will remove the water molecule and ketone will be formed. The product formed is Penan-2-one.
The reaction is given below:
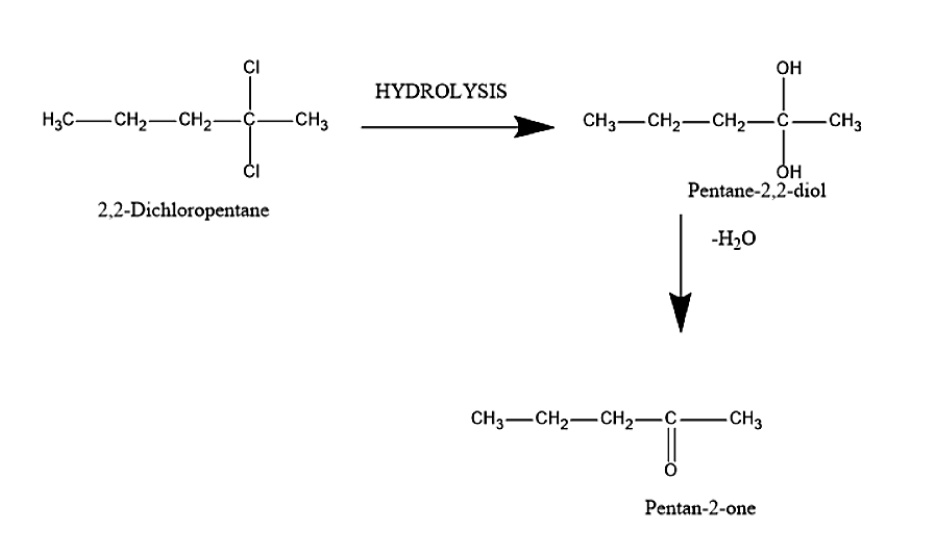
Therefore, the reactant used for the preparation of Pent-2-one is 2,2-Dichloropentane.
Hence the correct answer is option (A) 2,2-Dichloropentane.
Additional information:
Characteristics of Pentan-2-one are given below. They are:
- It is a colourless liquid.
- It has a pleasant ethereal odour.
- The flash point is \[45^\circ F\].
- It is present naturally in Nicotiana tabacum, which is also known as Tobacco.
- It is a less dense liquid which is soluble in water.
Notes: Ketones can be prepared from various methods such as:
- It can be prepared from acyl chloride.
- Oxidation of secondary alcohol.
- Dehydration of alkynes.
- Friedel crafts acylation.
- dehydrogenation of secondary alcohols.
Complete step by step answer:
Pentan-2-one is also known as methyl propyl ketone. Pent-2-one can be prepared from 2,2-Dichloropentane. In this reaction, the reactant contains two chlorine atoms. The reactant will undergo hydrolysis to give Pentane-2,2-diol. This happens when the chlorine atoms present in 2,2-Dichloropentane are replaced by the -OH group in order to give Pentane-2,2-diol. Hydration is a process which involves the addition of the water molecule.
Further the formed pentane-2,2-diol will undergo dehydration reaction. Dehydration is a process that involves removal of the water molecule. It will remove the water molecule and ketone will be formed. The product formed is Penan-2-one.
The reaction is given below:

Therefore, the reactant used for the preparation of Pent-2-one is 2,2-Dichloropentane.
Hence the correct answer is option (A) 2,2-Dichloropentane.
Additional information:
Characteristics of Pentan-2-one are given below. They are:
- It is a colourless liquid.
- It has a pleasant ethereal odour.
- The flash point is \[45^\circ F\].
- It is present naturally in Nicotiana tabacum, which is also known as Tobacco.
- It is a less dense liquid which is soluble in water.
Notes: Ketones can be prepared from various methods such as:
- It can be prepared from acyl chloride.
- Oxidation of secondary alcohol.
- Dehydration of alkynes.
- Friedel crafts acylation.
- dehydrogenation of secondary alcohols.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




