
Paracetamol is a/an:
(A) both antipyretic and analgesic
(B) analgesic
(C) antipyretic
(D) antimalarial
Answer
557.7k+ views
Hint: Paracetamol is the common name of acetaminophen. It consists of the core of a benzene ring on which one hydroxyl group is substituted along with nitrogen atom of the amide group on the para position of the ring. It is mildly given as a dose in the treatment of fever and pain.
Complete Step-by-step Answer
The amide group present on the ring is acetamide. The system is extensively conjugated as the $1$ lone pair is present on the oxygen of the hydroxyl group and the pi cloud of benzene and the lone pair on nitrogen.
The p orbital of the carbonyl carbon and a lone pair on the oxygen is also conjugated. The presence of $2$ activating groups makes the benzene ring more reactive toward the electrophilic aromatic substitution.
As the substituents are ortho-para directing and para concerning each other, all positions on the ring are equally activated. The conjugation also helps in reducing the basicity of the oxygen and the nitrogen and making the hydroxyl group acidic by delocalisation of charge developed on the phenoxide anion.
The structure of paracetamol is:
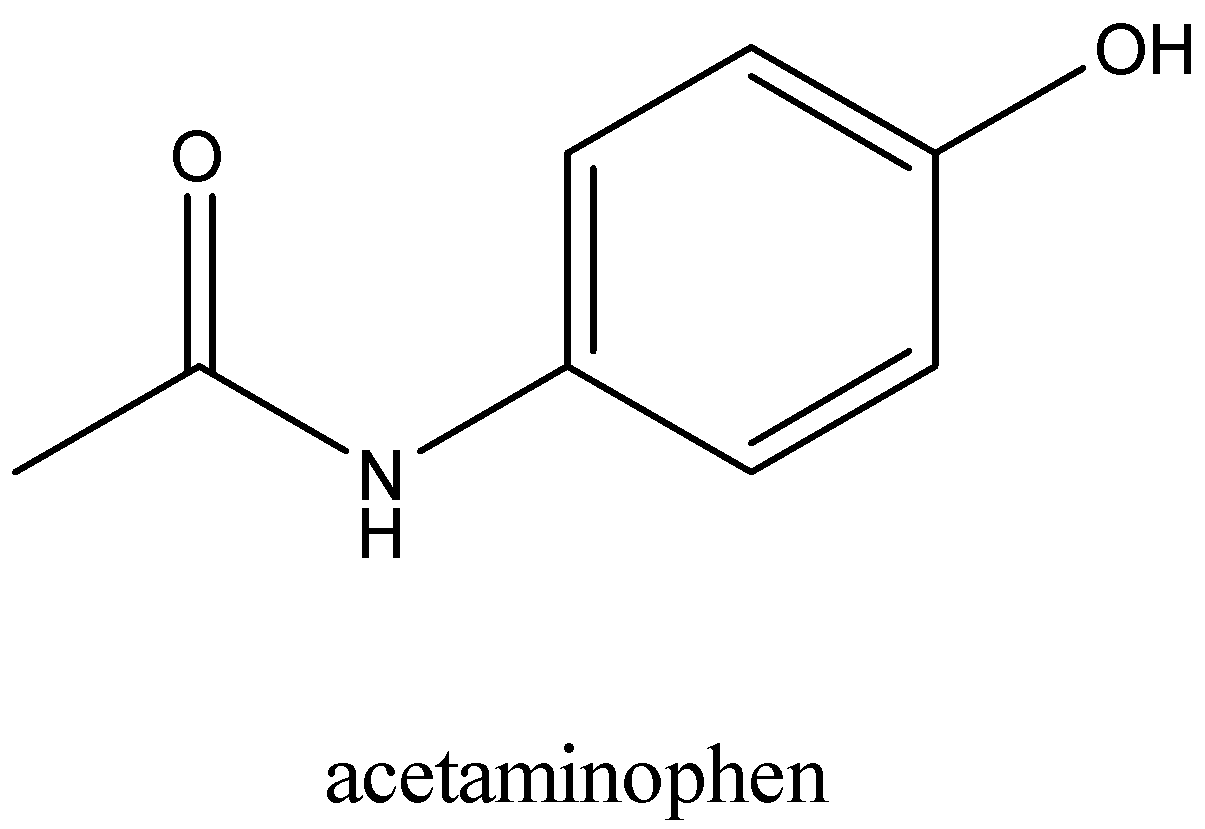
Paracetamol is used to relieve pain. It is given as a painkiller. It provides relief from pain without putting you to sleep or making you lose consciousness. Thus it works as an analgesic. It is also given as a pyretic drug in the treatment of fever.
Hence, Option (A) is correct.
Note:
Analgesics are the drugs that are given to kill aches and pain in the body without causing any drowsiness. Ibuprofen is one example of an analgesic. A Pyretic is a drug that is given to reduce the temperature of the body in fever. These drugs are a popular subcategory of NSAIDs.
Complete Step-by-step Answer
The amide group present on the ring is acetamide. The system is extensively conjugated as the $1$ lone pair is present on the oxygen of the hydroxyl group and the pi cloud of benzene and the lone pair on nitrogen.
The p orbital of the carbonyl carbon and a lone pair on the oxygen is also conjugated. The presence of $2$ activating groups makes the benzene ring more reactive toward the electrophilic aromatic substitution.
As the substituents are ortho-para directing and para concerning each other, all positions on the ring are equally activated. The conjugation also helps in reducing the basicity of the oxygen and the nitrogen and making the hydroxyl group acidic by delocalisation of charge developed on the phenoxide anion.
The structure of paracetamol is:

Paracetamol is used to relieve pain. It is given as a painkiller. It provides relief from pain without putting you to sleep or making you lose consciousness. Thus it works as an analgesic. It is also given as a pyretic drug in the treatment of fever.
Hence, Option (A) is correct.
Note:
Analgesics are the drugs that are given to kill aches and pain in the body without causing any drowsiness. Ibuprofen is one example of an analgesic. A Pyretic is a drug that is given to reduce the temperature of the body in fever. These drugs are a popular subcategory of NSAIDs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




