
Oxidation state of S in \[{H_2}{S_2}{O_8}\] is:
(A) +6
(B) +7
(C) +8
(D) 0
Answer
523k+ views
Hint: The name of the compound given in the question is Persulfuric acid. It contains peroxide linkage. The oxidation state of the oxygen atoms differ in case of peroxides from the normal oxidation state of the oxygen atoms.
Complete answer: The name of the compound given in the question is Persulfuric acid. As the name suggests, the compound has a peroxide group. Let’s see the chemical structure of \[{H_2}{S_2}{O_8}\] in order to find oxidation number of S.
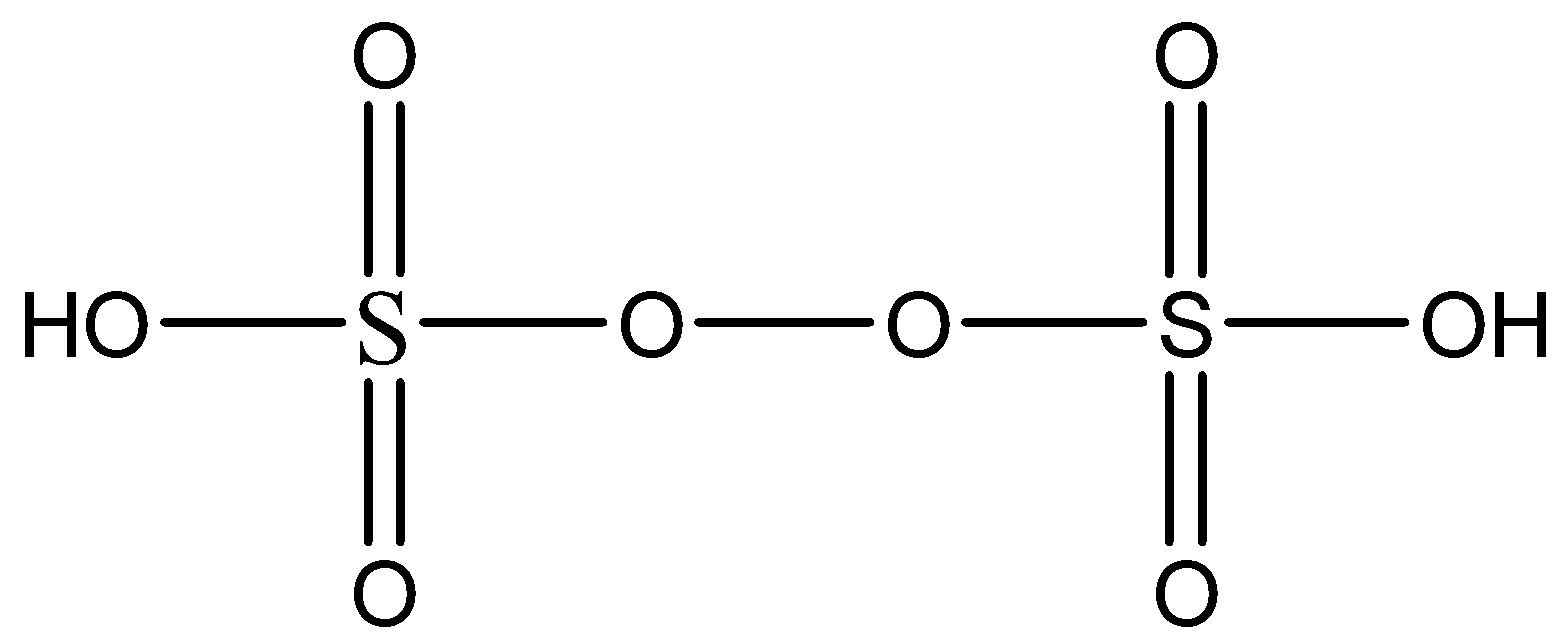
Now we know that we can find the oxidation number of any atom in a species by a fact that simply adding the oxidation numbers of all the atoms will give the overall charge on the species.
- Remember that both the oxygen atoms in the peroxide group are given an oxidation number of (-1). This is because each of this oxygen is bonded to another oxygen atom hence its oxidation number increases to (-1).
So, we can write that overall charge on \[{H_2}{S_2}{O_8}\] = 2(Oxidation number of S atom) + 2(Oxidation number of H atom) + 6(Oxidation number of normal oxygen atom) + 2(Oxidation number of peroxide oxygen)
We have multiplied the oxidation number of atoms according to the number of atoms present in the compound.
So, 0 = 2(Oxidation number of S atom) + 2(+1) + 6(-2) + 2(-1)
0 = 2(Oxidation number of S atom) + 2 - 12 -2
0 = 2(Oxidation number of S atom) - 12
2(Oxidation number of S atom) = 12
Oxidation number of S atom = +6
So, the correct answer is “Option A”.
Note:Do not consider that all the oxygen atoms have (-2) oxidation state in this compound. Even in the process of finding oxidation states of atoms in other compounds, make sure that you check the form of oxygen atoms, as they have different oxidation states if they are present as peroxide, superoxide or normal form.
Complete answer: The name of the compound given in the question is Persulfuric acid. As the name suggests, the compound has a peroxide group. Let’s see the chemical structure of \[{H_2}{S_2}{O_8}\] in order to find oxidation number of S.

Now we know that we can find the oxidation number of any atom in a species by a fact that simply adding the oxidation numbers of all the atoms will give the overall charge on the species.
- Remember that both the oxygen atoms in the peroxide group are given an oxidation number of (-1). This is because each of this oxygen is bonded to another oxygen atom hence its oxidation number increases to (-1).
So, we can write that overall charge on \[{H_2}{S_2}{O_8}\] = 2(Oxidation number of S atom) + 2(Oxidation number of H atom) + 6(Oxidation number of normal oxygen atom) + 2(Oxidation number of peroxide oxygen)
We have multiplied the oxidation number of atoms according to the number of atoms present in the compound.
So, 0 = 2(Oxidation number of S atom) + 2(+1) + 6(-2) + 2(-1)
0 = 2(Oxidation number of S atom) + 2 - 12 -2
0 = 2(Oxidation number of S atom) - 12
2(Oxidation number of S atom) = 12
Oxidation number of S atom = +6
So, the correct answer is “Option A”.
Note:Do not consider that all the oxygen atoms have (-2) oxidation state in this compound. Even in the process of finding oxidation states of atoms in other compounds, make sure that you check the form of oxygen atoms, as they have different oxidation states if they are present as peroxide, superoxide or normal form.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




