
Oxidation of cyclohexene in presence of acidic potassium permanganate leads to.
A.Glutaric acid
B.Adipic acid
C.Pimelic acid
D.Succinic acid
Answer
581.4k+ views
Hint: Potassium permanganate under mild conditions can affect conversion of alkenes to glycols. However, it is capable of further oxidizing the glycol with cleavage of the carbon-carbon \[\left( {C - C} \right)\] bond, so careful control of the reaction conditions is necessary. A cyclic manganese diester is an intermediate in these oxidations, which results in glycols formed by syn addition.
Complete step by step answer:
The cleavage of double bonds by oxidation is useful in the synthesis of acids and ketones and determining structures. There are various methods which are available including ozonolysis and hot concentrated permanganate. The products obtained from it depend on the original structure of the olefin. The equations below illustrate the products from cleavage of alkenes:
The balanced equation for the oxidation of cyclohexene with permanganate is shown:
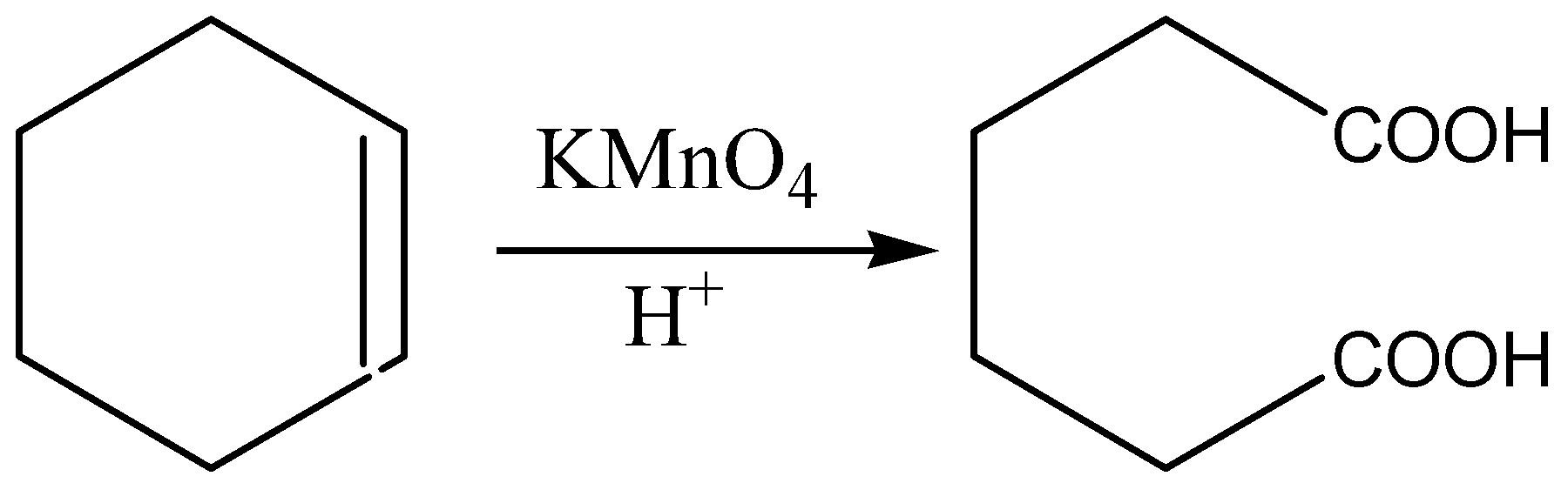
When the reaction of cyclohexene with hot KMnO4 happens, Oxidative cleavage takes place. The double bond is broken to which oxygen atoms are going to be added forming a carboxylic acid group at each. Thus, the cyclic structure is broken, forming hexan-1,6-dioic acid i.e., adipic acid.
The oxidation of cyclohexene in the presence of acidic potassium permanganate \[\left( {KMn{O_4}} \right)\] leads to Adipic acid which is hexane dioic acid. The \[C = C\] double bond in cyclohexene is broken down and the C atoms that are attached to the double bond are oxidized to \[ - COOH\] groups.
Therefore, the correct answer is option (B).
Note: Adipic acid or hexane dioic acid is the organic compound having the formula \[{\left( {C{H_2}} \right)_4}{\left( {COOH} \right)_2}\]. Adipic acid from an industrial perspective is the most important dicarboxylic acid as about 2.5 billion kg of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. This acid otherwise rarely occurs in nature.
Complete step by step answer:
The cleavage of double bonds by oxidation is useful in the synthesis of acids and ketones and determining structures. There are various methods which are available including ozonolysis and hot concentrated permanganate. The products obtained from it depend on the original structure of the olefin. The equations below illustrate the products from cleavage of alkenes:
The balanced equation for the oxidation of cyclohexene with permanganate is shown:

When the reaction of cyclohexene with hot KMnO4 happens, Oxidative cleavage takes place. The double bond is broken to which oxygen atoms are going to be added forming a carboxylic acid group at each. Thus, the cyclic structure is broken, forming hexan-1,6-dioic acid i.e., adipic acid.
The oxidation of cyclohexene in the presence of acidic potassium permanganate \[\left( {KMn{O_4}} \right)\] leads to Adipic acid which is hexane dioic acid. The \[C = C\] double bond in cyclohexene is broken down and the C atoms that are attached to the double bond are oxidized to \[ - COOH\] groups.
Therefore, the correct answer is option (B).
Note: Adipic acid or hexane dioic acid is the organic compound having the formula \[{\left( {C{H_2}} \right)_4}{\left( {COOH} \right)_2}\]. Adipic acid from an industrial perspective is the most important dicarboxylic acid as about 2.5 billion kg of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. This acid otherwise rarely occurs in nature.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




