
What is the oxidation number for sulfur?
Answer
495.6k+ views
Hint: For this question, we have to know about the oxidation number of an atom. The oxidation state of sulphur is according to the compound formed. As Sulphur is bigger in size so it has an empty $d$ orbital due to which it can expand its valances. So it exhibits $ - 2$ , $ + 2$ ,$ + 4$ and $ + 6$ oxidation states respectively.
Complete answer:
Oxidation number which is also termed as oxidation state is the total number of electrons that an atom gains or loses in the formation of a chemical bond with another atom, basically it simplifies the process of determining what is being oxidized or reduced in the redox reactions. Example, The oxidation number of $S{O_4}^{2 - }$ ion is $ + 6$ .
Sulphur has various oxidation states because of its bigger size. Sulphur has an empty $d$ orbital which may be used in bonding, and they can form four or six bonds by unpaired electrons.
S atom in ground state:
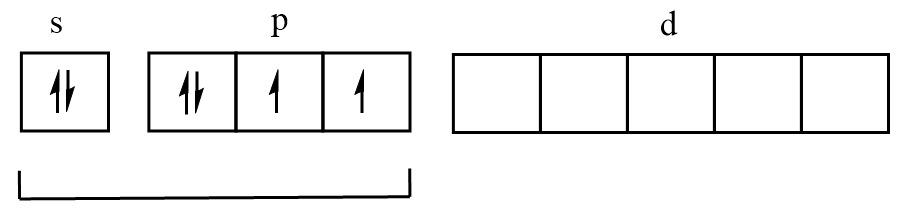
Two unpaired electrons therefore can form two bonds and four electron pairs.
Excited state:
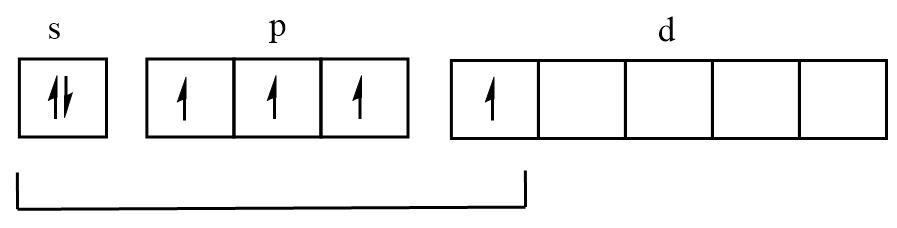
Four unpaired electrons therefore can form four bonds and five electron pairs.
Further excited state:
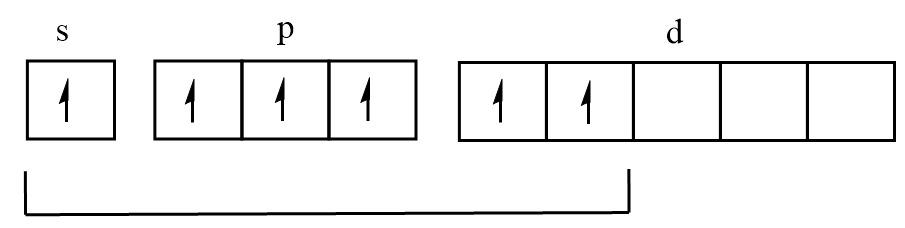
Six unpaired electrons therefore can form six bonds and six electron pairs.
For example:
1.In $S{O_4}^{2 - }$ , let $x$ be the oxidation number of $S$ in $S{O_4}^{2 - }$
The oxidation number of $O$ is $ - 2$ . So, the oxidation number of S is:
$x + 4 \times ( - 2) = - 2$
$ \Rightarrow x - 8 = - 2$
$ \Rightarrow x = + 6$
2.In ${H_2}S{O_3}$ , let $x$ be the oxidation number of $S$ in ${H_2}S{O_3}$
The oxidation number of $O$ is $ - 2$, for hydrogen is $ + 1$ . So, the oxidation number of S is:
$2 \times 1 + x + 3 \times ( - 2) = 0$
$ \Rightarrow 2 + x - 6 = 0$
$ \Rightarrow x = + 4$
Note:
Compounds of Sulphur are generally tetravalent. The $( + IV)$ state shows both oxidizing and reducing properties. Compounds in the $( + VI)$ state show oxidizing properties. The higher oxidation states become less stable on the descending group. These compounds are typically volatile because they are covalent compounds.
Complete answer:
Oxidation number which is also termed as oxidation state is the total number of electrons that an atom gains or loses in the formation of a chemical bond with another atom, basically it simplifies the process of determining what is being oxidized or reduced in the redox reactions. Example, The oxidation number of $S{O_4}^{2 - }$ ion is $ + 6$ .
Sulphur has various oxidation states because of its bigger size. Sulphur has an empty $d$ orbital which may be used in bonding, and they can form four or six bonds by unpaired electrons.
S atom in ground state:

Two unpaired electrons therefore can form two bonds and four electron pairs.
Excited state:

Four unpaired electrons therefore can form four bonds and five electron pairs.
Further excited state:

Six unpaired electrons therefore can form six bonds and six electron pairs.
For example:
1.In $S{O_4}^{2 - }$ , let $x$ be the oxidation number of $S$ in $S{O_4}^{2 - }$
The oxidation number of $O$ is $ - 2$ . So, the oxidation number of S is:
$x + 4 \times ( - 2) = - 2$
$ \Rightarrow x - 8 = - 2$
$ \Rightarrow x = + 6$
2.In ${H_2}S{O_3}$ , let $x$ be the oxidation number of $S$ in ${H_2}S{O_3}$
The oxidation number of $O$ is $ - 2$, for hydrogen is $ + 1$ . So, the oxidation number of S is:
$2 \times 1 + x + 3 \times ( - 2) = 0$
$ \Rightarrow 2 + x - 6 = 0$
$ \Rightarrow x = + 4$
Note:
Compounds of Sulphur are generally tetravalent. The $( + IV)$ state shows both oxidizing and reducing properties. Compounds in the $( + VI)$ state show oxidizing properties. The higher oxidation states become less stable on the descending group. These compounds are typically volatile because they are covalent compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




