
O-nitrophenol has lower boiling point than p-nitrophenol. Why?
Answer
574.2k+ views
Hint: The boiling point of a compound depends on the strength of bonds it has. If bonds are very strong, for example, if compounds have strong hydrogen bonding, then it will require more energy for the compound to break the bond and their boiling point will be more too.
Complete answer:
The boiling point of a liquid varies depending upon the encompassing environmental pressure. A liquid in a very partial vacuum features a boiling point not up to when that liquid is at air pressure. A liquid at an air mass includes a higher boiling point than when that liquid is kept at air pressure.
Large molecules have more electrons and nuclei that make van der Waals attractive forces, so their compounds usually have higher boiling points than similar compounds made from smaller molecules. It's important to use this rule only to check the boiling point of compounds.
Boiling point of ortho nitrophenol is below p-nitrophenol, this is often due to their structures that result in differing kinds of hydrogen bonding.
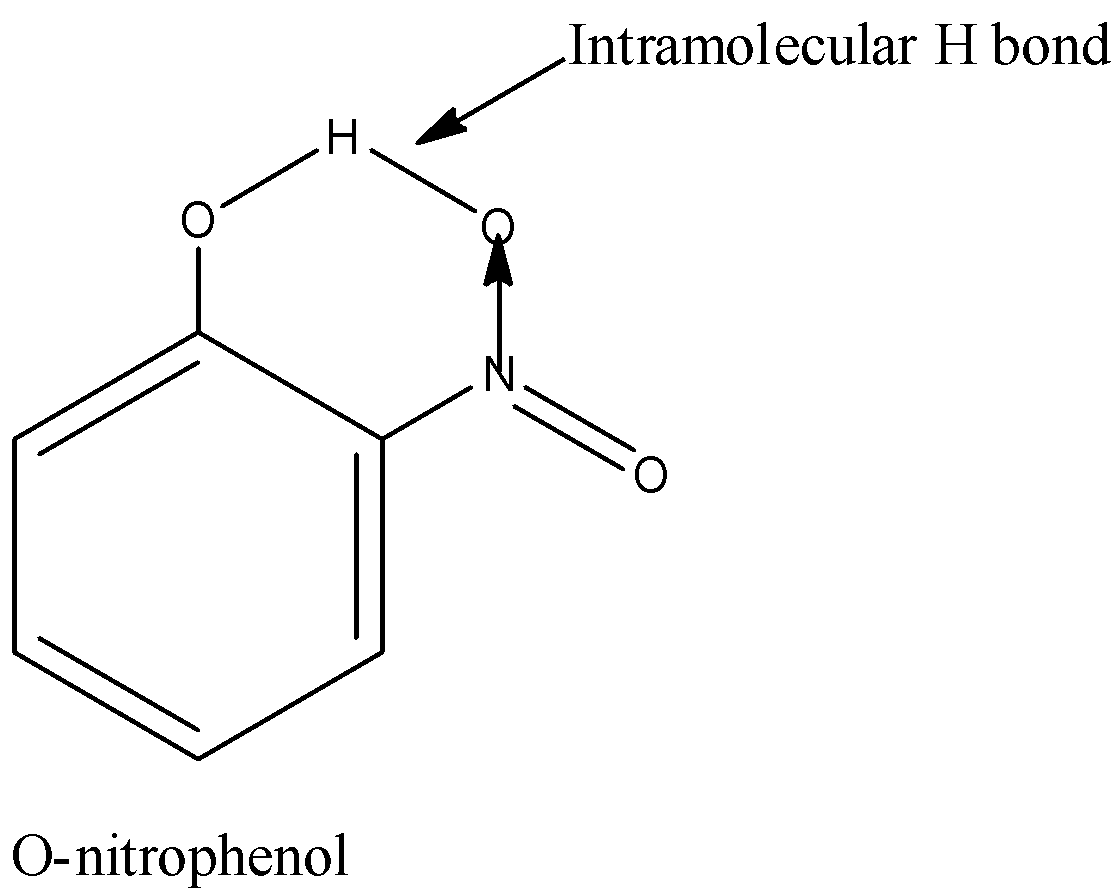
Lets know first about hydrogen bonding: Hydrogen bonding could be a sort of electrostatic attraction between an electronegative element and H-atom attached to an electronegative element [keep it in mind every hydrogen cannot participate in H-bonding; only that H attached to an electronegative element will participate in H-bonding
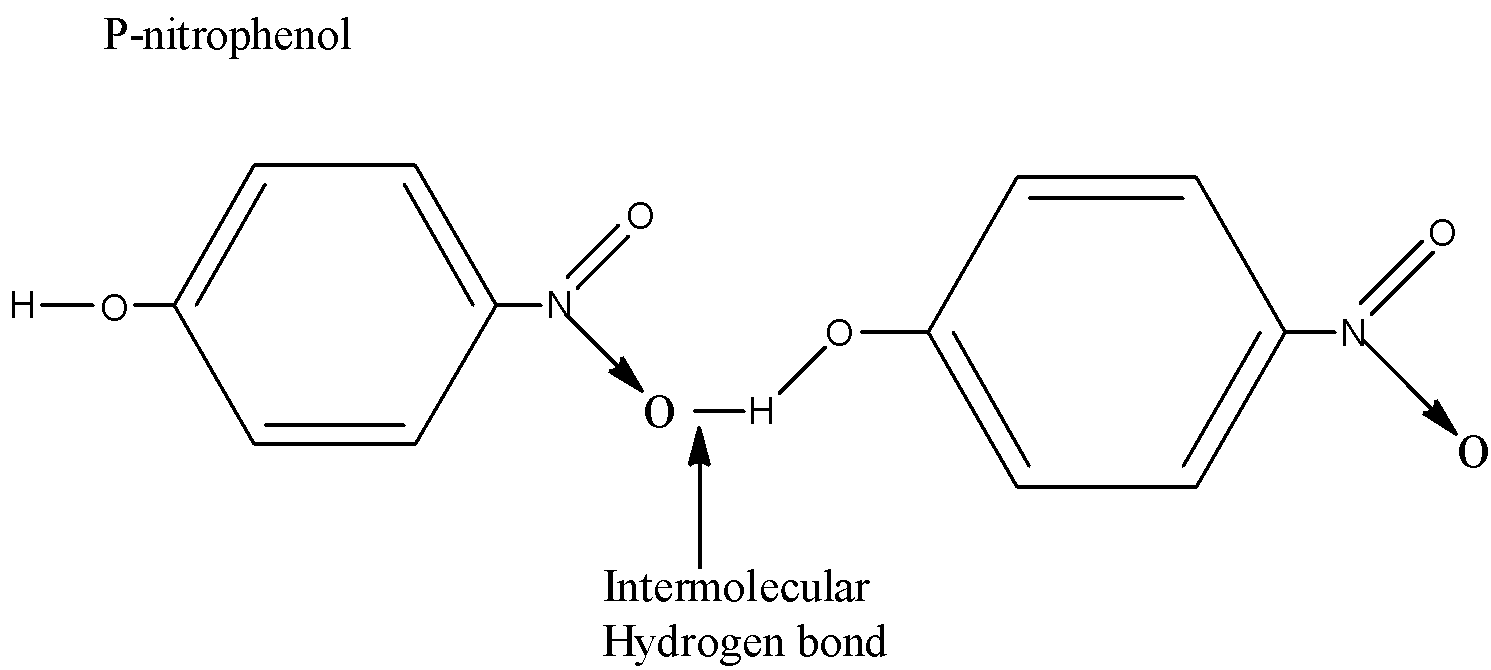
Now if the hydrogen bonding acts within a molecule, we call it as intramolecular hydrogen bonding; but if it acts within molecules, we call it as intermolecular hydrogen bonding
Whereas for para nitrophenol there's the possibility of intermolecular(within molecules) hydrogen bonding.
Hence just in case of o-nitrophenol, thanks to intramolecular H-bonding it acts as a monomer whereas for para isomers thanks to intermolecular H-bonding it's ready to keep company with other molecules and acts as polymer-like. Thus the mass of the system increases just in case of para isomer and hence the boiling point also increases.
Hence, Boiling point of Ortho nitrophenol is not up to p-nitrophenol.
Let's summarize the solution:
P-nitrophenol has intermolecular hydrogen bonding. Intermolecular hydrogen bonding results in a molecular association. This increases boiling point. Hence, O-nitrophenol contains a lower boiling point than P-nitrophenol.
So here is the answer.
Note:
Remember that para compounds have the maximum boiling point, out of meta and ortho isomers. It is so because para compounds are very stable due to their plane of symmetry. There might be some exceptions, the only reason being hydrogen bonding.
Complete answer:
The boiling point of a liquid varies depending upon the encompassing environmental pressure. A liquid in a very partial vacuum features a boiling point not up to when that liquid is at air pressure. A liquid at an air mass includes a higher boiling point than when that liquid is kept at air pressure.
Large molecules have more electrons and nuclei that make van der Waals attractive forces, so their compounds usually have higher boiling points than similar compounds made from smaller molecules. It's important to use this rule only to check the boiling point of compounds.
Boiling point of ortho nitrophenol is below p-nitrophenol, this is often due to their structures that result in differing kinds of hydrogen bonding.


Lets know first about hydrogen bonding: Hydrogen bonding could be a sort of electrostatic attraction between an electronegative element and H-atom attached to an electronegative element [keep it in mind every hydrogen cannot participate in H-bonding; only that H attached to an electronegative element will participate in H-bonding
Now if the hydrogen bonding acts within a molecule, we call it as intramolecular hydrogen bonding; but if it acts within molecules, we call it as intermolecular hydrogen bonding
Whereas for para nitrophenol there's the possibility of intermolecular(within molecules) hydrogen bonding.
Hence just in case of o-nitrophenol, thanks to intramolecular H-bonding it acts as a monomer whereas for para isomers thanks to intermolecular H-bonding it's ready to keep company with other molecules and acts as polymer-like. Thus the mass of the system increases just in case of para isomer and hence the boiling point also increases.
Hence, Boiling point of Ortho nitrophenol is not up to p-nitrophenol.
Let's summarize the solution:
P-nitrophenol has intermolecular hydrogen bonding. Intermolecular hydrogen bonding results in a molecular association. This increases boiling point. Hence, O-nitrophenol contains a lower boiling point than P-nitrophenol.
So here is the answer.
Note:
Remember that para compounds have the maximum boiling point, out of meta and ortho isomers. It is so because para compounds are very stable due to their plane of symmetry. There might be some exceptions, the only reason being hydrogen bonding.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




