
One of allotrope of oxygen is ozone. It is a pale-blue poisonous gas with strong odour.
A. True
B. False
Answer
567k+ views
Hint: There are many known allotropes of oxygen. One of the allotrope of oxygen is allotrope. Ozone is a highly toxic gas and acts as a sunblock for our earth. Chemical formula for ozone is \[{O_3}\] . Ozone acts as a layer in the Stratosphere where Ozone concentration is high. Ozone is found in the stratosphere which is $10 - 50Km$ above the ground.
Complete step by step answer:
The upper Stratosphere consists of high concentration of ozone \[\left( {{O_3}} \right)\], which protects our life and covers the whole planet from the harmful ultraviolet (UV) radiation coming from the sun towards the earth. Ozone \[\left( {{O_3}} \right)\] is present in upper stratosphere and obtained as a byproduct of ultraviolet (UV) radiation acting on oxygen molecules \[\left( {{O_2}} \right)\] .
Now first we will discuss the formation of ozone \[\left( {{O_3}} \right)\]. First step is that the UV radiation breaks molecular oxygen \[\left( {{O_2}} \right)\] into free oxygen \[\left( O \right)\] atoms. The first step can be written as follows:
${O_2}(g)\xrightarrow{{UV}}O(g) + O(g)$
The next step is that oxygen \[\left( O \right)\] atoms will combine with oxygen molecules \[\left( {{O_2}} \right)\] to form ozone oxygen molecules \[\left( {{O_3}} \right)\].
\[O(g) + {O_2}(g)\overset {UV} \leftrightarrows {O_3}(g)\]
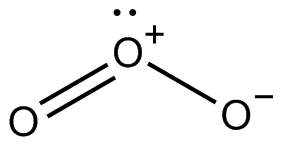
When an ozone \[\left( {{O_3}} \right)\] molecule absorbs UV radiation it breaks in a molecular oxygen \[\left( {{O_2}} \right)\] and free oxygen \[\left( O \right)\] atoms. The free oxygen \[\left( O \right)\] atom obtained again combines with molecular oxygen \[\left( {{O_2}} \right)\] to form ozone\[\left( {{O_3}} \right)\]. This cycle is called an ‘Oxygen-Ozone’ cycle and due to this cycle harmful ultraviolet (UV) radiations are continuously absorbed as heat.
Final Result: One of allotrope of oxygen is ozone. It is a pale-blue poisonous gas with strong odour.
So, the statement is true.
Note: The Ozone \[\left( {{O_3}} \right)\] molecule absorbs ultraviolet (UV) rays between $310$ to $210$ nm wavelength. chlorofluorocarbon compounds (CFCs), nitrogen oxides, chlorine, tetrachloride are ozone depleting substances (ODS). More than \[1000ppm\] of ozone leads to dizziness and headache.
Complete step by step answer:
The upper Stratosphere consists of high concentration of ozone \[\left( {{O_3}} \right)\], which protects our life and covers the whole planet from the harmful ultraviolet (UV) radiation coming from the sun towards the earth. Ozone \[\left( {{O_3}} \right)\] is present in upper stratosphere and obtained as a byproduct of ultraviolet (UV) radiation acting on oxygen molecules \[\left( {{O_2}} \right)\] .
Now first we will discuss the formation of ozone \[\left( {{O_3}} \right)\]. First step is that the UV radiation breaks molecular oxygen \[\left( {{O_2}} \right)\] into free oxygen \[\left( O \right)\] atoms. The first step can be written as follows:
${O_2}(g)\xrightarrow{{UV}}O(g) + O(g)$
The next step is that oxygen \[\left( O \right)\] atoms will combine with oxygen molecules \[\left( {{O_2}} \right)\] to form ozone oxygen molecules \[\left( {{O_3}} \right)\].
\[O(g) + {O_2}(g)\overset {UV} \leftrightarrows {O_3}(g)\]

When an ozone \[\left( {{O_3}} \right)\] molecule absorbs UV radiation it breaks in a molecular oxygen \[\left( {{O_2}} \right)\] and free oxygen \[\left( O \right)\] atoms. The free oxygen \[\left( O \right)\] atom obtained again combines with molecular oxygen \[\left( {{O_2}} \right)\] to form ozone\[\left( {{O_3}} \right)\]. This cycle is called an ‘Oxygen-Ozone’ cycle and due to this cycle harmful ultraviolet (UV) radiations are continuously absorbed as heat.
Final Result: One of allotrope of oxygen is ozone. It is a pale-blue poisonous gas with strong odour.
So, the statement is true.
Note: The Ozone \[\left( {{O_3}} \right)\] molecule absorbs ultraviolet (UV) rays between $310$ to $210$ nm wavelength. chlorofluorocarbon compounds (CFCs), nitrogen oxides, chlorine, tetrachloride are ozone depleting substances (ODS). More than \[1000ppm\] of ozone leads to dizziness and headache.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




