
On the hydrolysis of ${R_3}SiCl$, forms
A.${R_3}SiOH$
B.${R_3}Si - O - Si{R_3}$
C.${R_2}Si = O$
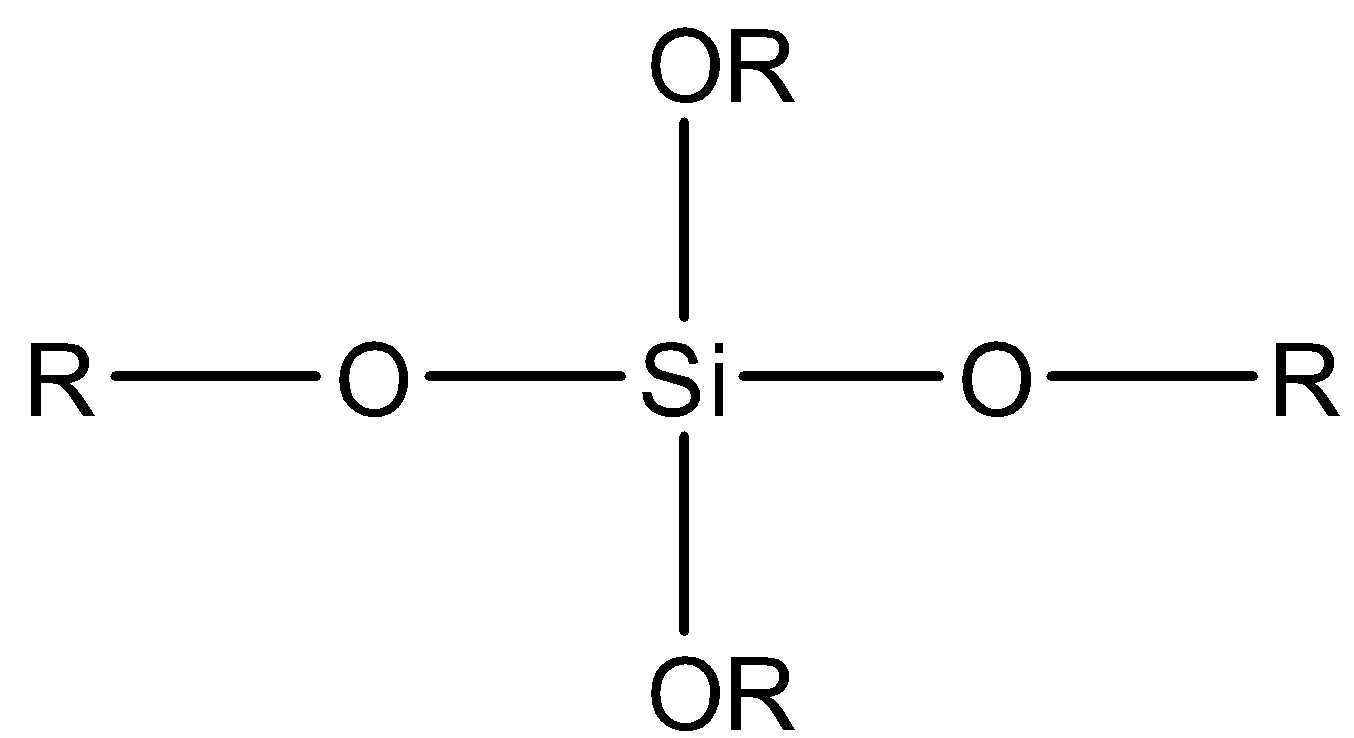
D.

Answer
571.8k+ views
Hint: We can say a hydrolysis is the reaction in which a chemical bond present in the substrate is broken by its reaction with water. Usually, hydroxyl groups of water get linked to the electropositive atom and hydrogen present in water along with electronegative atoms forms a bond.
Complete step by step solution:
We know that hydrolysis reaction is a reaction in which a molecule of water breaks the bond in the substrate molecule. Now according to the question, ${R_3}SiCl$ is the substrate molecule.
$Si - Cl$ bond is the most polar bond here. This is due to the highly electronegative element which is the chlorine. The electronegativity of silicon is similar to that of the carbon. So, we can say an atom of chlorine contains partial negative charge and a partial positive charge is present on silicon atom.
In water, $O{H^ - }$ is the nucleophilic part and the electrophilic part is ${H^ + }$.
The mechanism could be given as,
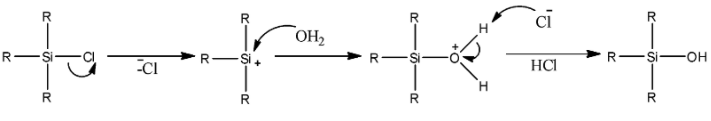
We can see that a cation is formed first. The cation gets nucleophilic attack by oxygen atoms of water. A silicon compound containing hydroxyl groups is formed. So, ${R_3}SiOH$ is the final product.
We can write the chemical reaction as,
${R_3}SiCl\xrightarrow{{{H_2}O}}{R_3}SiOH + HCl$
${R_3}SiOH + {R_3}SiOH\xrightarrow[{ - {H_2}O}]{{Condensation}}{R_3}Si - O - Si{R_3}$
The hydrolysis product of ${R_3}SiCl$ is ${R_3}SiOH$. Option (1) is correct.
When we condense the product ${R_3}SiOH$, the product formed will be ${R_3}Si - O - Si{R_3}$ and molecules of water. Option (2) is also correct.
So, we can say ${R_3}SiCl$ on hydrolysis reaction forms ${R_3}SiOH$.
And hence option 1 and 2 is correct.
Note:We have to remember that the common formula of aryl (or) acyl substituted silicon chlorides are ${R_n}SiC{l_{4 - n}}$ and they are known as silicones. We can use silicones as electrical insulators, sealants, and in the manufacture of grease and waterproofing fabrics.
Complete step by step solution:
We know that hydrolysis reaction is a reaction in which a molecule of water breaks the bond in the substrate molecule. Now according to the question, ${R_3}SiCl$ is the substrate molecule.
$Si - Cl$ bond is the most polar bond here. This is due to the highly electronegative element which is the chlorine. The electronegativity of silicon is similar to that of the carbon. So, we can say an atom of chlorine contains partial negative charge and a partial positive charge is present on silicon atom.
In water, $O{H^ - }$ is the nucleophilic part and the electrophilic part is ${H^ + }$.
The mechanism could be given as,

We can see that a cation is formed first. The cation gets nucleophilic attack by oxygen atoms of water. A silicon compound containing hydroxyl groups is formed. So, ${R_3}SiOH$ is the final product.
We can write the chemical reaction as,
${R_3}SiCl\xrightarrow{{{H_2}O}}{R_3}SiOH + HCl$
${R_3}SiOH + {R_3}SiOH\xrightarrow[{ - {H_2}O}]{{Condensation}}{R_3}Si - O - Si{R_3}$
The hydrolysis product of ${R_3}SiCl$ is ${R_3}SiOH$. Option (1) is correct.
When we condense the product ${R_3}SiOH$, the product formed will be ${R_3}Si - O - Si{R_3}$ and molecules of water. Option (2) is also correct.
So, we can say ${R_3}SiCl$ on hydrolysis reaction forms ${R_3}SiOH$.
And hence option 1 and 2 is correct.
Note:We have to remember that the common formula of aryl (or) acyl substituted silicon chlorides are ${R_n}SiC{l_{4 - n}}$ and they are known as silicones. We can use silicones as electrical insulators, sealants, and in the manufacture of grease and waterproofing fabrics.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




