
On the addition of which compound the reaction will proceed in the forward direction?
$[B{(OH)_3} + NaOH \to Na[B{(OH)_4}] $ (aq)\]
A) $Cis - 1,2 - diol$
B) $Trans - 1,2 - diol$
C) $Borax$
D) $N{a_2}HP{O_4}$
Answer
564.9k+ views
Hint: This reaction will be considered as a reversible reaction because when sodium metaborate $Na[B(O{H_4})]$ is formed by the reaction between $B{(OH)_3}$ and $NaOH$ takes place. This sodium metaborate gets hydrolyzed and moves the reaction in the backward direction thereby regenerating $B{(OH)_3}$ and $NaOH$ .
The backward reaction is given as:
$Na[B{(OH)_4}] \to NaOH + B{(OH)_3}$ (on hydrolysis)
Complete solution:
If we add certain organic polyhydroxy compounds such as glycerol, $Cis - 1,2 - diol$ , catechol, mannitol or sugars to the reaction mixture, then $B{(OH)_3}$ will act as a strong monobasic acid and will further combine with polyhydroxy compounds to give chelated complex compound. The chelated complex compound formed will increase the stability of the complex and due to this higher stability, the reaction will proceed in the forward direction.
So, the added compound must be $Cis - 1,2 - diol$ to enhance the properties of the acid present. It will thus form a very stable complex with ${[B{(OH)_4}]^ - }$ thereby removing it from the solution. We already know that the reaction is a reversible reaction and so on the removal of this chelated complex formed, will shift the reaction in the forward direction. And thus all the $B{(OH)_3}$ present in the reaction mixture will react with $NaOH$ which also means that it will behave as a strong acid in the presence of $Cis - 1,2 - diol$.
The chelated complex formed is:
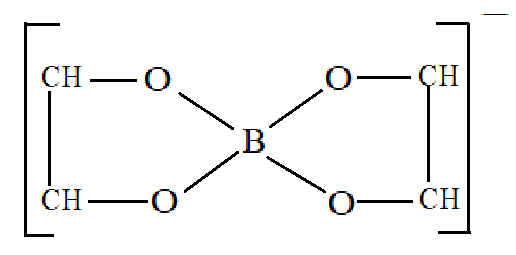
Therefore, from the above explanation we can say that the correct option is (A).
Note: We know that a chelate complex is a structure which is formed when a central metal atom binds with large molecules known as ligands in a cyclic or ring structure. Chelate complexes are more stable because they contain multidentate ligands which are more stable than unidentate ligands. Multidentate ligands are more stable because they can displace more than one molecule of water.
The backward reaction is given as:
$Na[B{(OH)_4}] \to NaOH + B{(OH)_3}$ (on hydrolysis)
Complete solution:
If we add certain organic polyhydroxy compounds such as glycerol, $Cis - 1,2 - diol$ , catechol, mannitol or sugars to the reaction mixture, then $B{(OH)_3}$ will act as a strong monobasic acid and will further combine with polyhydroxy compounds to give chelated complex compound. The chelated complex compound formed will increase the stability of the complex and due to this higher stability, the reaction will proceed in the forward direction.
So, the added compound must be $Cis - 1,2 - diol$ to enhance the properties of the acid present. It will thus form a very stable complex with ${[B{(OH)_4}]^ - }$ thereby removing it from the solution. We already know that the reaction is a reversible reaction and so on the removal of this chelated complex formed, will shift the reaction in the forward direction. And thus all the $B{(OH)_3}$ present in the reaction mixture will react with $NaOH$ which also means that it will behave as a strong acid in the presence of $Cis - 1,2 - diol$.
The chelated complex formed is:

Therefore, from the above explanation we can say that the correct option is (A).
Note: We know that a chelate complex is a structure which is formed when a central metal atom binds with large molecules known as ligands in a cyclic or ring structure. Chelate complexes are more stable because they contain multidentate ligands which are more stable than unidentate ligands. Multidentate ligands are more stable because they can displace more than one molecule of water.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




