
Of the following, which is the product formed when cyclohexanone undergoes aldol condensation followed by heating?
A.

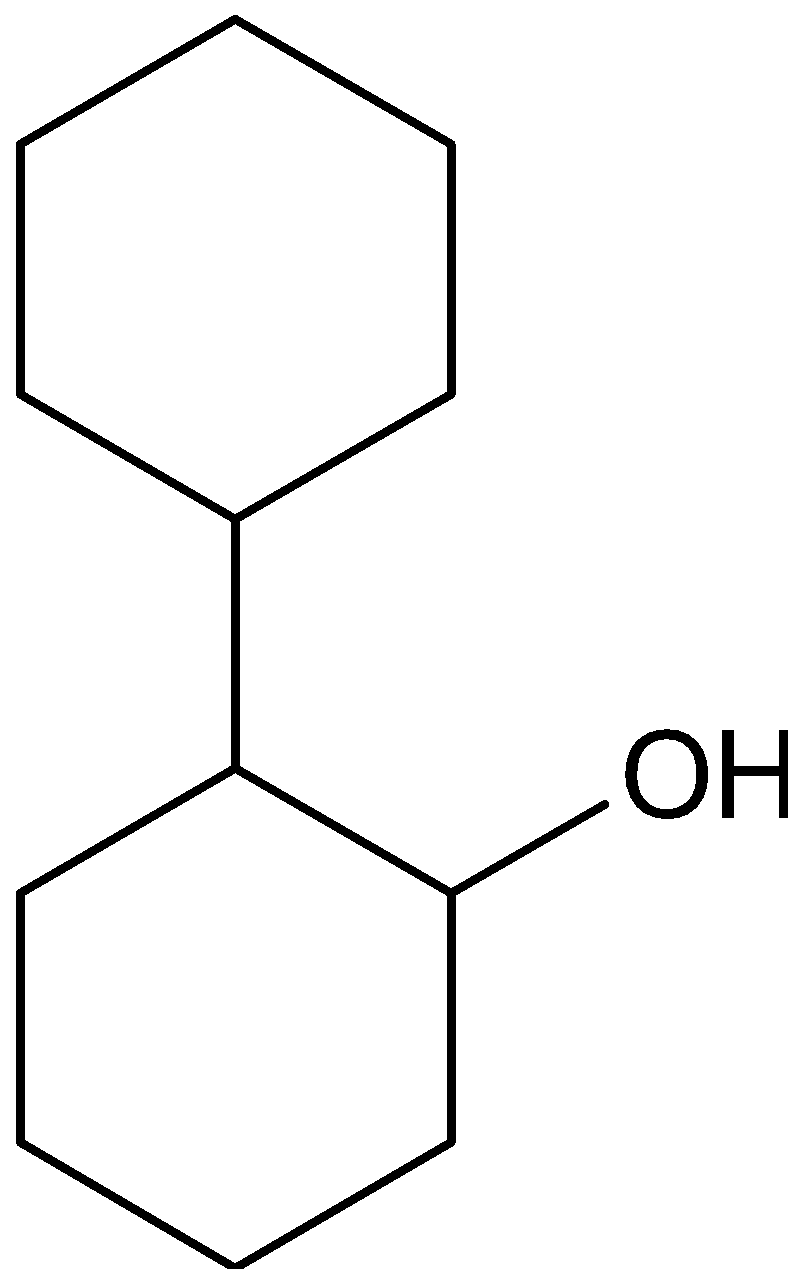
B.
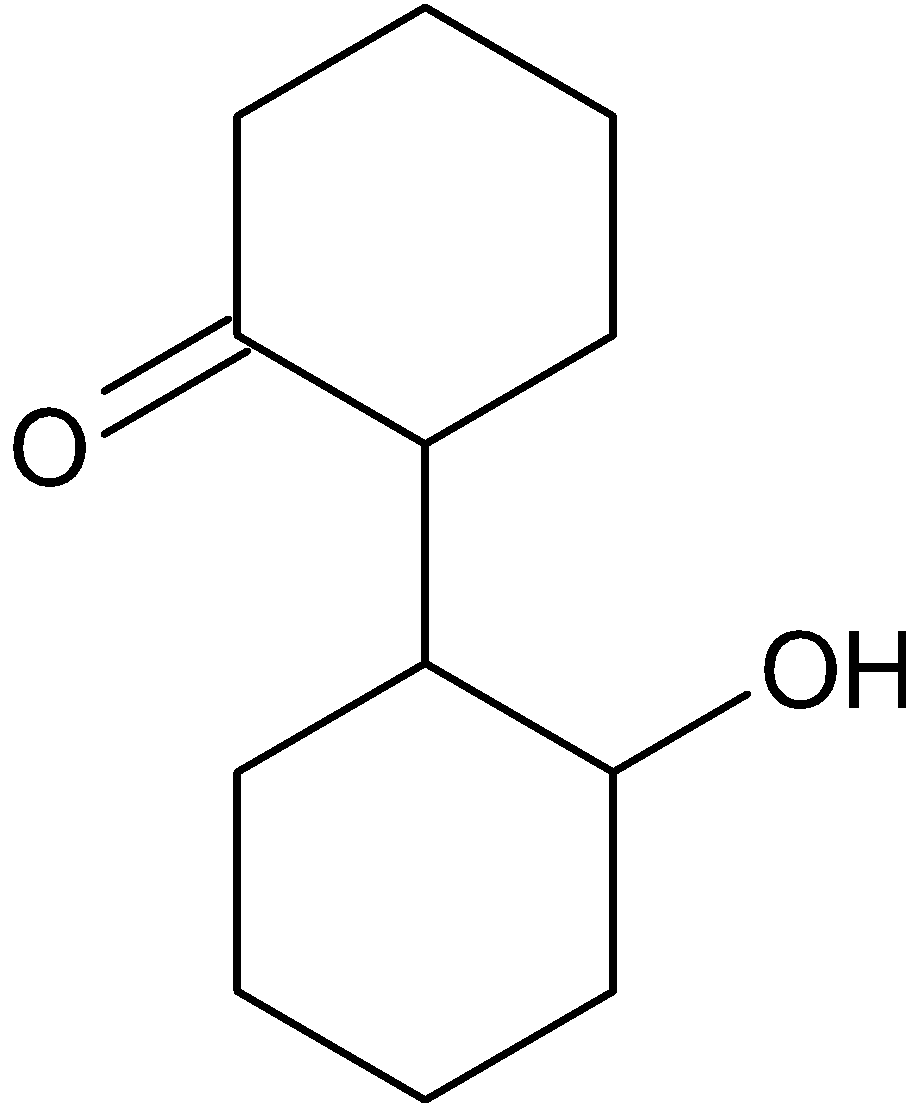
C.
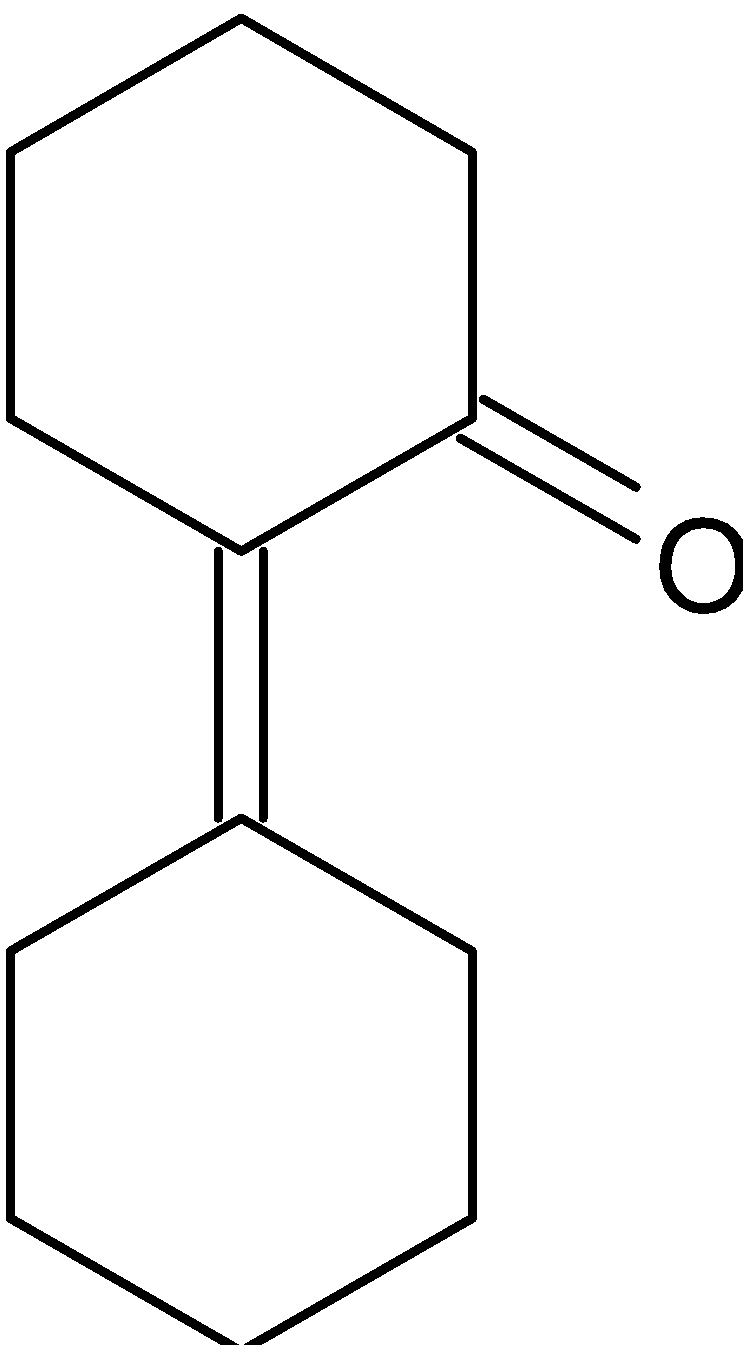
D.




Answer
593.7k+ views
Hint:Condensation between two molecules of an aldehyde or ketone to form a $\beta - $ hydroxy aldehyde or a $\beta - $ hydroxy ketone is called aldol condensation. It is possible only when the carbonyl compound contains at least one $\alpha - $ hydrogen atom.
Complete step by step answer:
Aldol condensation is a property of carbonyl compounds having hydrogen at $\alpha - $ carbon atom. Aldol means aldehyde and alcohol groups on the same molecule. It may occur between two aldehydes or ketones in the presence of a catalytic base. The reaction is only possible between two components having $\alpha - $ hydrogen.
When cyclohexanone undergoes aldol condensation in the presence of a base, it will produce a $\beta - $ hydroxy ketone.
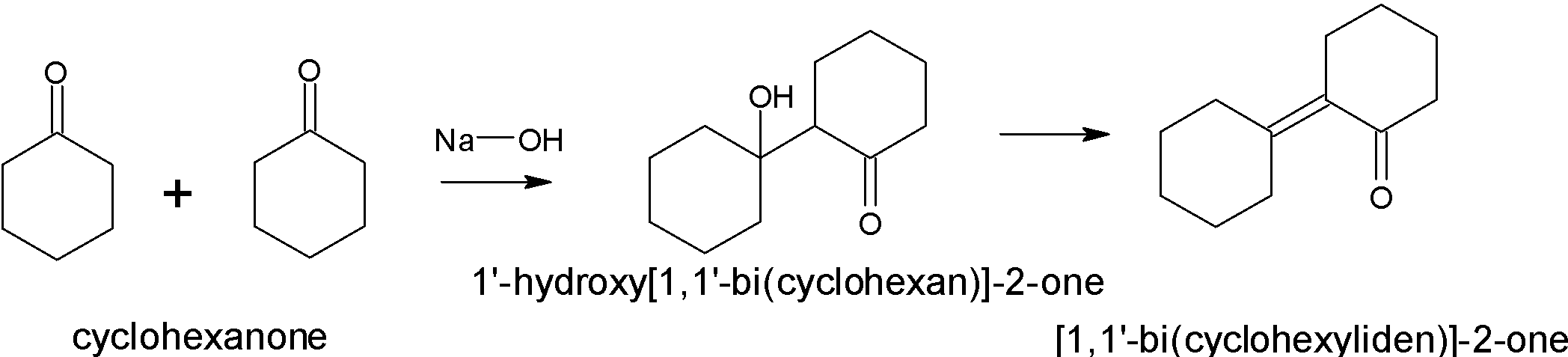
The mechanism involves three steps:
-First is the acid-base reaction. Hydroxide functions as a base and removes the acidic $\alpha - $ hydrogen giving the reactive enolate.
-The nucleophilic enolate anion attacks the ketone at the electrophilic carbonyl carbon in a nucleophilic addition giving an intermediate alkoxide. Alkoxide ion is the conjugate base of alcohols.
-The alkoxide deprotonates a water molecule creating hydroxide and the aldol is formed.
-Addition of water results in the formation of condensation products.
-Water molecules are removed by heating in the presence of acid. This makes a double bond.
Thus the product formed is a $\alpha ,\beta - $ unsaturated alkene.
Hence the correct option is C.
Note:
Some conditions regarding the aldol condensation are:
A reversible equilibrium
${\text{OH}}$ is the base typically used in an aldol reaction.
Can be carried out either by aldehydes or ketones.
With aldehydes, the equilibrium favors products.
With ketones, the equilibrium favors the reactants.
Complete step by step answer:
Aldol condensation is a property of carbonyl compounds having hydrogen at $\alpha - $ carbon atom. Aldol means aldehyde and alcohol groups on the same molecule. It may occur between two aldehydes or ketones in the presence of a catalytic base. The reaction is only possible between two components having $\alpha - $ hydrogen.
When cyclohexanone undergoes aldol condensation in the presence of a base, it will produce a $\beta - $ hydroxy ketone.

The mechanism involves three steps:
-First is the acid-base reaction. Hydroxide functions as a base and removes the acidic $\alpha - $ hydrogen giving the reactive enolate.
-The nucleophilic enolate anion attacks the ketone at the electrophilic carbonyl carbon in a nucleophilic addition giving an intermediate alkoxide. Alkoxide ion is the conjugate base of alcohols.
-The alkoxide deprotonates a water molecule creating hydroxide and the aldol is formed.
-Addition of water results in the formation of condensation products.
-Water molecules are removed by heating in the presence of acid. This makes a double bond.
Thus the product formed is a $\alpha ,\beta - $ unsaturated alkene.
Hence the correct option is C.
Note:
Some conditions regarding the aldol condensation are:
A reversible equilibrium
${\text{OH}}$ is the base typically used in an aldol reaction.
Can be carried out either by aldehydes or ketones.
With aldehydes, the equilibrium favors products.
With ketones, the equilibrium favors the reactants.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




