
How will you obtain
A. Diacetone amine from acetone
B. Urotropine from formaldehyde
C. Acetaldoxime from acetaldehyde
Answer
541.5k+ views
Hint: We should know the structure of the acetone, formaldehyde and acetaldehyde to prepare the diacetone, urotropine and acetaldoxime. The structures of acetone, formaldehyde and acetaldehyde are as follows.
\[\underset{ACETONE}{\mathop{C{{H}_{3}}COC{{H}_{3}}}}\,,\underset{FORMALDEHYDE}{\mathop{HCHO}}\,,\underset{ACETALDEHYDE}{\mathop{C{{H}_{3}}CHO}}\,\]
Complete answer:
- In the question it is given to write the preparation of diacetone amine from acetone, urotropine from formaldehyde and acetaldoxime and acetaldehyde.
a) The preparation of diacetone amine from acetone is as follows.
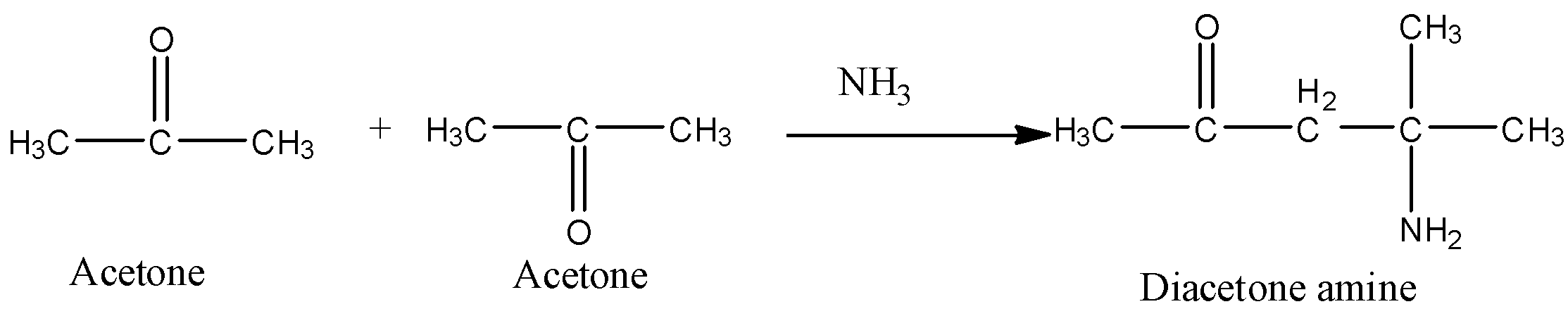
- In the above chemical reaction two moles of acetone are going to react with one mole of ammonia and form one mole of diacetone amine as the product.
- The diacetone amine is going to form by the condensation of 2 moles of acetone with ammonia.
b) The preparation of Urotropine from formaldehyde is as follows.
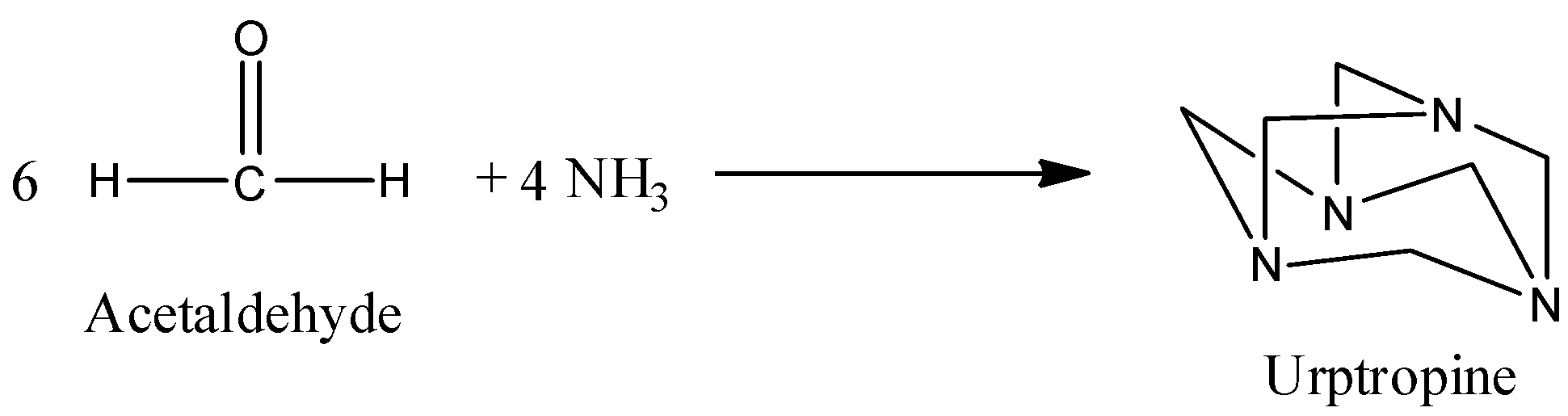
- In the above chemical reaction 6 moles of formaldehyde reacts with four moles of ammonia and forms one mole of urotropine as the product.
c) The preparation of Acetaldoxime from acetaldehyde is as follows.
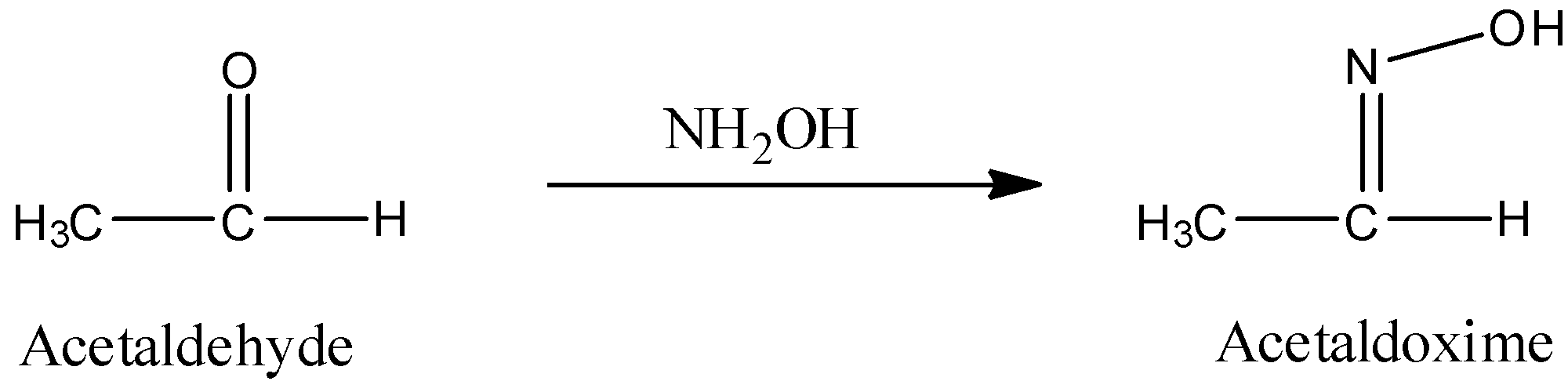
- In the above chemical reaction one mole of acetaldehyde is going to react with hydroxyl amine and forms one mole of the acetaldoxime as the product.
Note:
Methenamine is also called urotropine and it is a cage like structure. Urotropine is going to look like adamantine. The salt form of methanamine is going to be used to treat the urinary tract infection disease. Acetaldoxime is useful in boilers to act as an oxygen scavenger.
\[\underset{ACETONE}{\mathop{C{{H}_{3}}COC{{H}_{3}}}}\,,\underset{FORMALDEHYDE}{\mathop{HCHO}}\,,\underset{ACETALDEHYDE}{\mathop{C{{H}_{3}}CHO}}\,\]
Complete answer:
- In the question it is given to write the preparation of diacetone amine from acetone, urotropine from formaldehyde and acetaldoxime and acetaldehyde.
a) The preparation of diacetone amine from acetone is as follows.

- In the above chemical reaction two moles of acetone are going to react with one mole of ammonia and form one mole of diacetone amine as the product.
- The diacetone amine is going to form by the condensation of 2 moles of acetone with ammonia.
b) The preparation of Urotropine from formaldehyde is as follows.
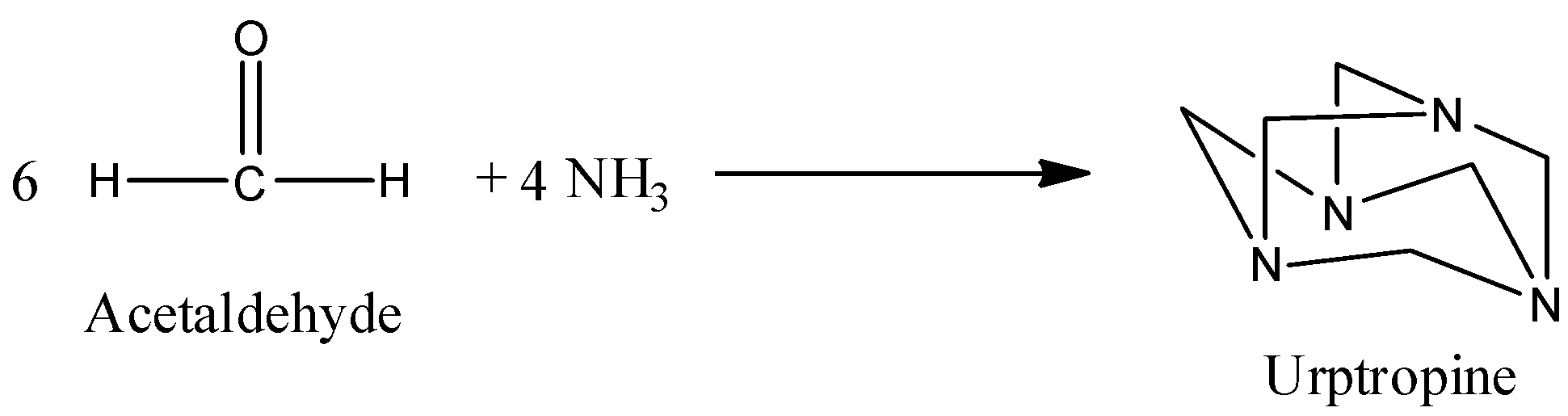
- In the above chemical reaction 6 moles of formaldehyde reacts with four moles of ammonia and forms one mole of urotropine as the product.
c) The preparation of Acetaldoxime from acetaldehyde is as follows.

- In the above chemical reaction one mole of acetaldehyde is going to react with hydroxyl amine and forms one mole of the acetaldoxime as the product.
Note:
Methenamine is also called urotropine and it is a cage like structure. Urotropine is going to look like adamantine. The salt form of methanamine is going to be used to treat the urinary tract infection disease. Acetaldoxime is useful in boilers to act as an oxygen scavenger.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




