
Observe the diagram, state and explain under which category this chemical reaction falls.

Answer
548.7k+ views
Hint: Limestone is most commonly known as calcium carbonate. It is kind of carbonate sedimentary rock, mainly composed of minerals like calcite and aragonite.
Complete answer:
- Limestone is an extensively versatile mineral. It is a carbonated sedimentary rock formed on the seafloor where materials rich in calcium carbonate get accumulated.
- Limestone is also known as calcium carbonate since it is the main component.
- It is even a part of the diet of terrestrial animals and is added in the diet of poultry animals to improve egg quality.
- When limestone undergoes metamorphism, it crystallizes to form marble.
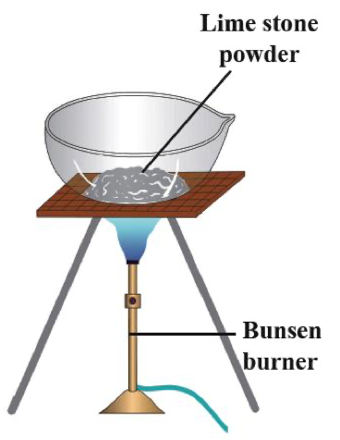
- The given diagram shows strong heating of limestone power.
- As limestone is heated, the calcium carbonate present inside it absorbs heat. This indicates the reaction proceeds as an endothermic reaction.
- After heating, limestone granules breakdown and decompose to form calcium oxide (quick lime).
- Reaction involved is shown below
\begin{align*}
CaCO_{3}(s)\xrightarrow{\Delta }CaO(s)+CO_{2}(g)
\end{align*}
- This reaction is a type of thermal decomposition and the process is known as calcining.
Additional information:
Marine organisms like coral leave their calcium carbonate shells behind as they die. This is the reason why 10% of the sedimentary rocks are limestones.
Note: Limestone is soluble in water and weak acids. When mixed with water, it gives slaked lime. It even contains variable amounts of silica in the form of chert.
Complete answer:
- Limestone is an extensively versatile mineral. It is a carbonated sedimentary rock formed on the seafloor where materials rich in calcium carbonate get accumulated.
- Limestone is also known as calcium carbonate since it is the main component.
- It is even a part of the diet of terrestrial animals and is added in the diet of poultry animals to improve egg quality.
- When limestone undergoes metamorphism, it crystallizes to form marble.
- The given diagram shows strong heating of limestone power.
- As limestone is heated, the calcium carbonate present inside it absorbs heat. This indicates the reaction proceeds as an endothermic reaction.
- After heating, limestone granules breakdown and decompose to form calcium oxide (quick lime).
- Reaction involved is shown below
\begin{align*}
CaCO_{3}(s)\xrightarrow{\Delta }CaO(s)+CO_{2}(g)
\end{align*}
- This reaction is a type of thermal decomposition and the process is known as calcining.
Additional information:
Marine organisms like coral leave their calcium carbonate shells behind as they die. This is the reason why 10% of the sedimentary rocks are limestones.
Note: Limestone is soluble in water and weak acids. When mixed with water, it gives slaked lime. It even contains variable amounts of silica in the form of chert.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




