
How many numbers of electrons are involved in the formation of a nitrogen molecule?
A. Three
B. Four
C. Eight
D. Six
Answer
573.6k+ views
Hint: Try to first explain about the electronic configuration of nitrogen atoms and then recall the structural formula of a nitrogen molecule. Nitrogen is a non-metal and it has five electrons in its outer shell.
Complete answer:
We know that the atomic number of nitrogen is $7$ and the electronic configuration of nitrogen is $2,5$ which means two electrons are present in s orbital and five electrons in p orbital. The five electrons that are present in the p-orbital are present in the outermost shell. Thus, these electrons are considered as the valence electrons. To attain a stable state either five electrons are donated or three electrons are gained by the nitrogen atom.
When two nitrogen atoms will react, three electrons (from five valence electrons) of each nitrogen atom are shared between them so that both will obtain a stable configuration. By sharing six electrons where the outer shell touches each nitrogen atom will count eight electrons (stable configuration). The full outer shells with the shared electrons are now stable forming a covalent bond and this can be shown through the following diagram:
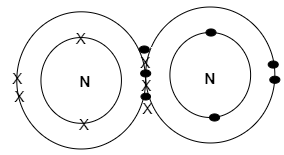
Thus, in total six electrons are involved in the formation of a nitrogen molecule (${{N}_{2}}$).
Hence, the correct option is D.
Note: Molecular nitrogen (${{N}_{2}}$) is a very common chemical compound in which two nitrogen atoms are tightly bound together by a strong covalent bond. Molecular nitrogen is a colorless, odorless, tasteless and inert gas at standard temperature and pressure.
Complete answer:
We know that the atomic number of nitrogen is $7$ and the electronic configuration of nitrogen is $2,5$ which means two electrons are present in s orbital and five electrons in p orbital. The five electrons that are present in the p-orbital are present in the outermost shell. Thus, these electrons are considered as the valence electrons. To attain a stable state either five electrons are donated or three electrons are gained by the nitrogen atom.
When two nitrogen atoms will react, three electrons (from five valence electrons) of each nitrogen atom are shared between them so that both will obtain a stable configuration. By sharing six electrons where the outer shell touches each nitrogen atom will count eight electrons (stable configuration). The full outer shells with the shared electrons are now stable forming a covalent bond and this can be shown through the following diagram:

Thus, in total six electrons are involved in the formation of a nitrogen molecule (${{N}_{2}}$).
Hence, the correct option is D.
Note: Molecular nitrogen (${{N}_{2}}$) is a very common chemical compound in which two nitrogen atoms are tightly bound together by a strong covalent bond. Molecular nitrogen is a colorless, odorless, tasteless and inert gas at standard temperature and pressure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




