
Number of compounds which are aromatic $ = w$
A. Number of compounds which are non-aromatic $ = x$
B. Number of compounds which are anti-aromatic $ = y$
C. Number of compounds which are readily undergo Dimerization at room temperature $ = z$
Sum of $w + x + y + z = ....$
Among the following compound
Compound Compound Compound (a)
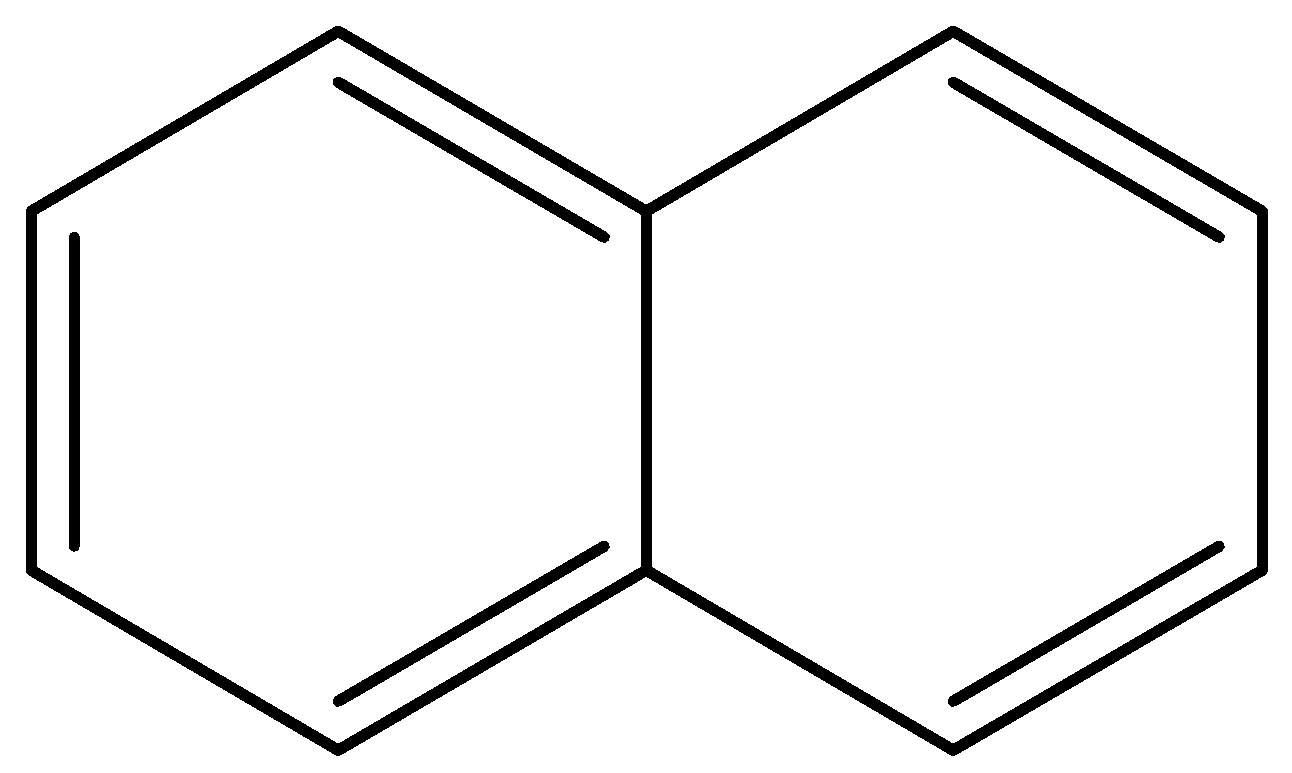
(b) ${C_8}H_8^{ - 2}$ (c)
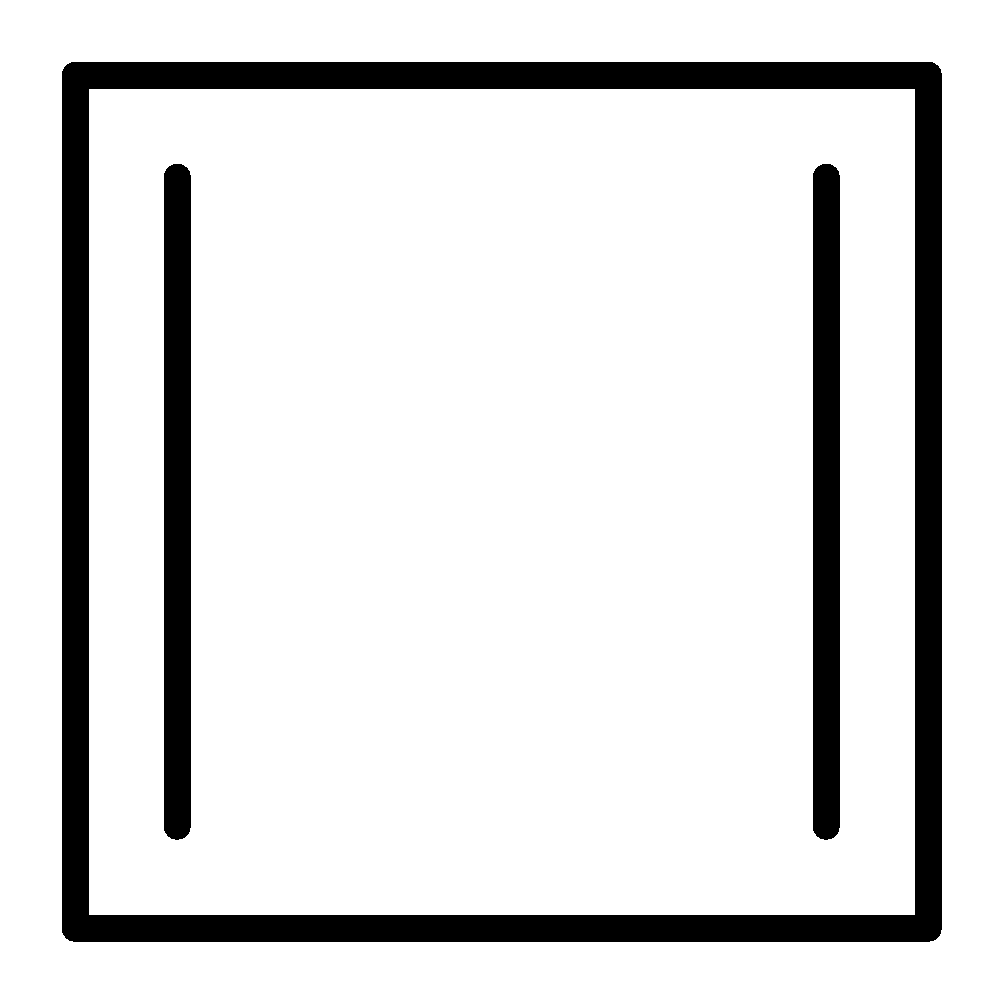
(d)
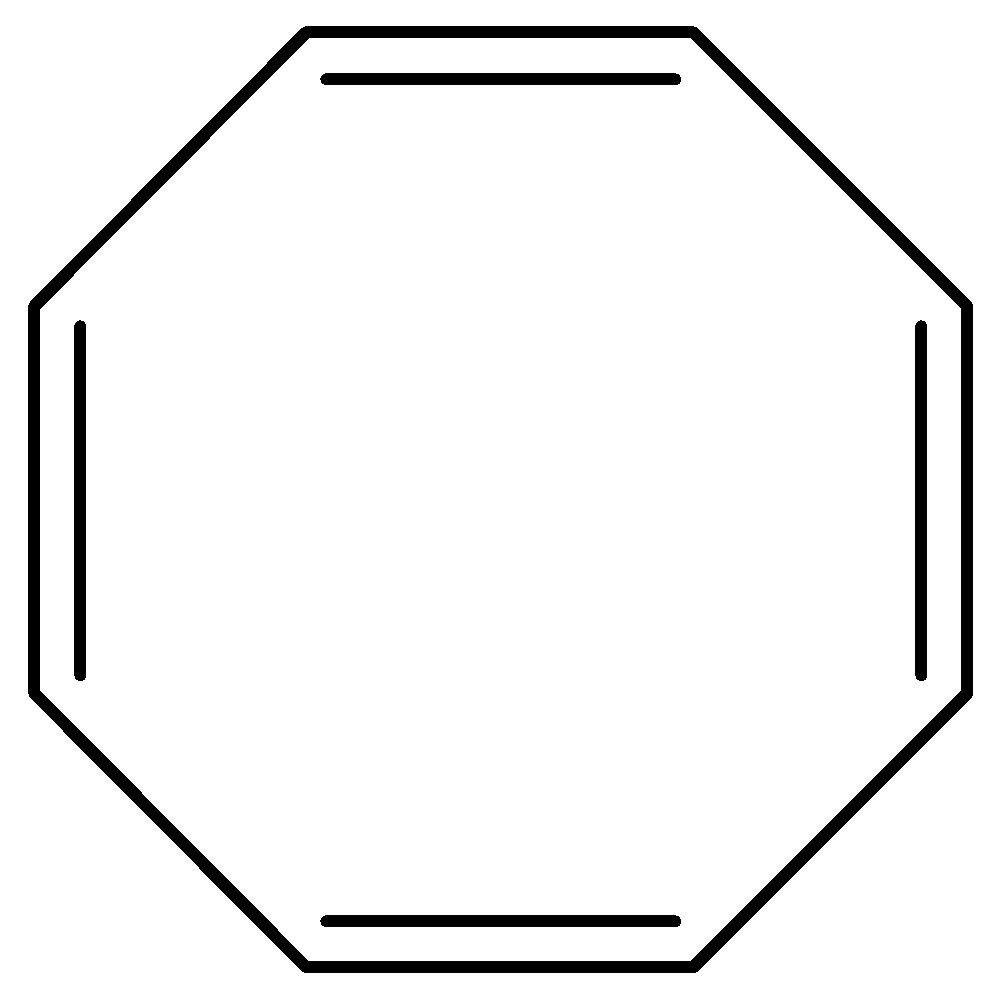
(e)
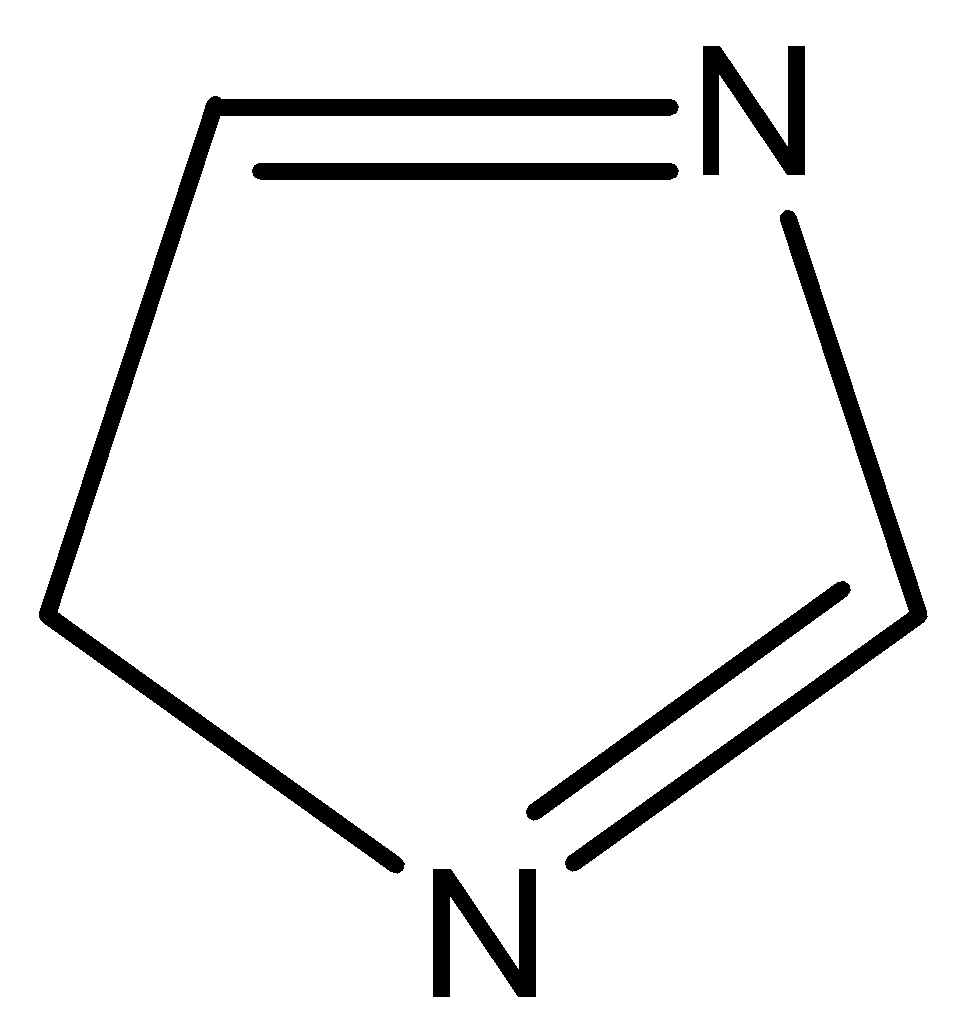
(f)
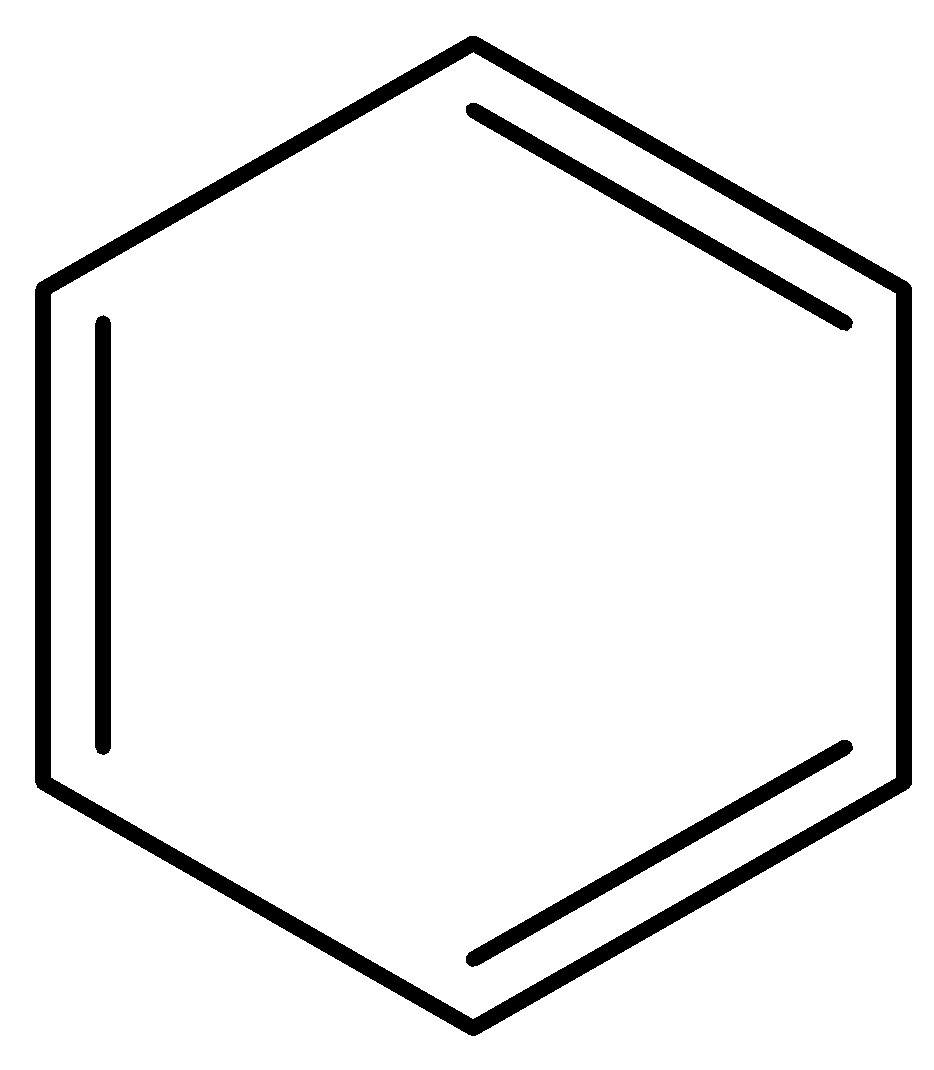
(g)
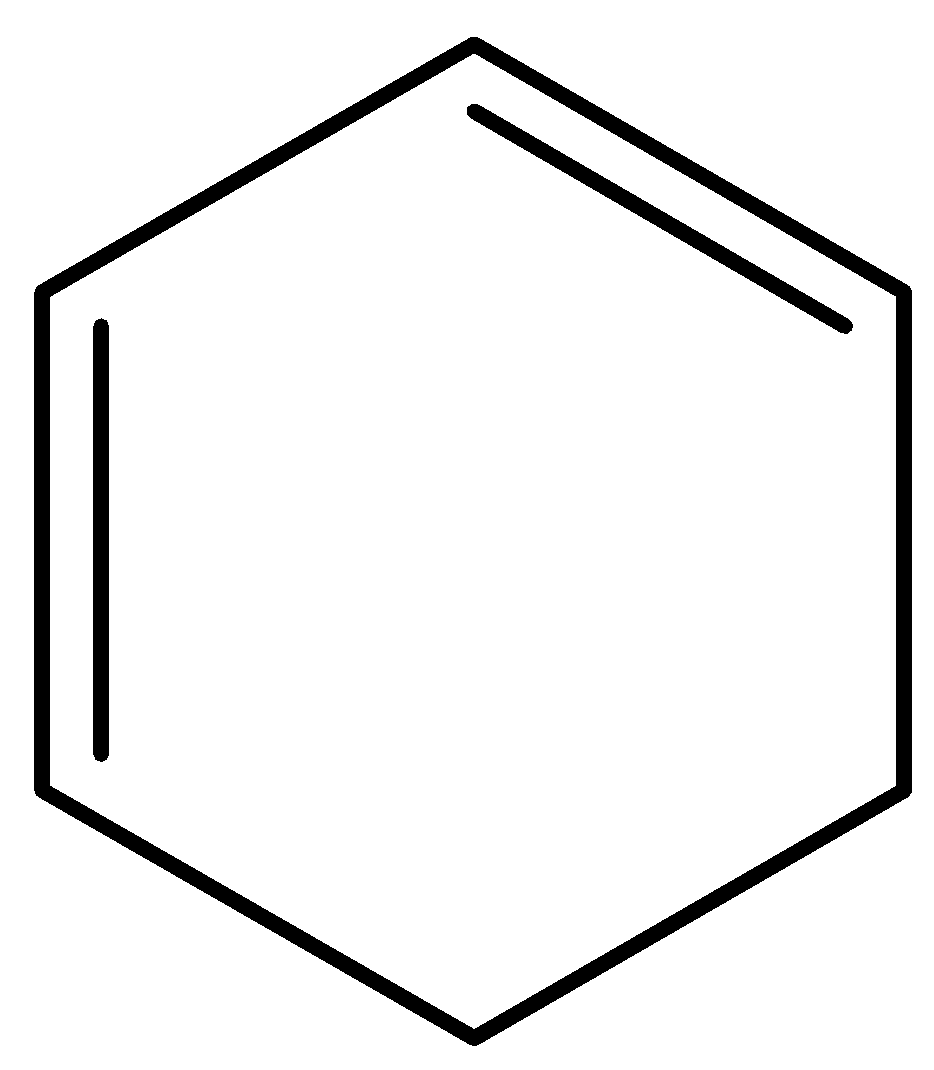
(h)
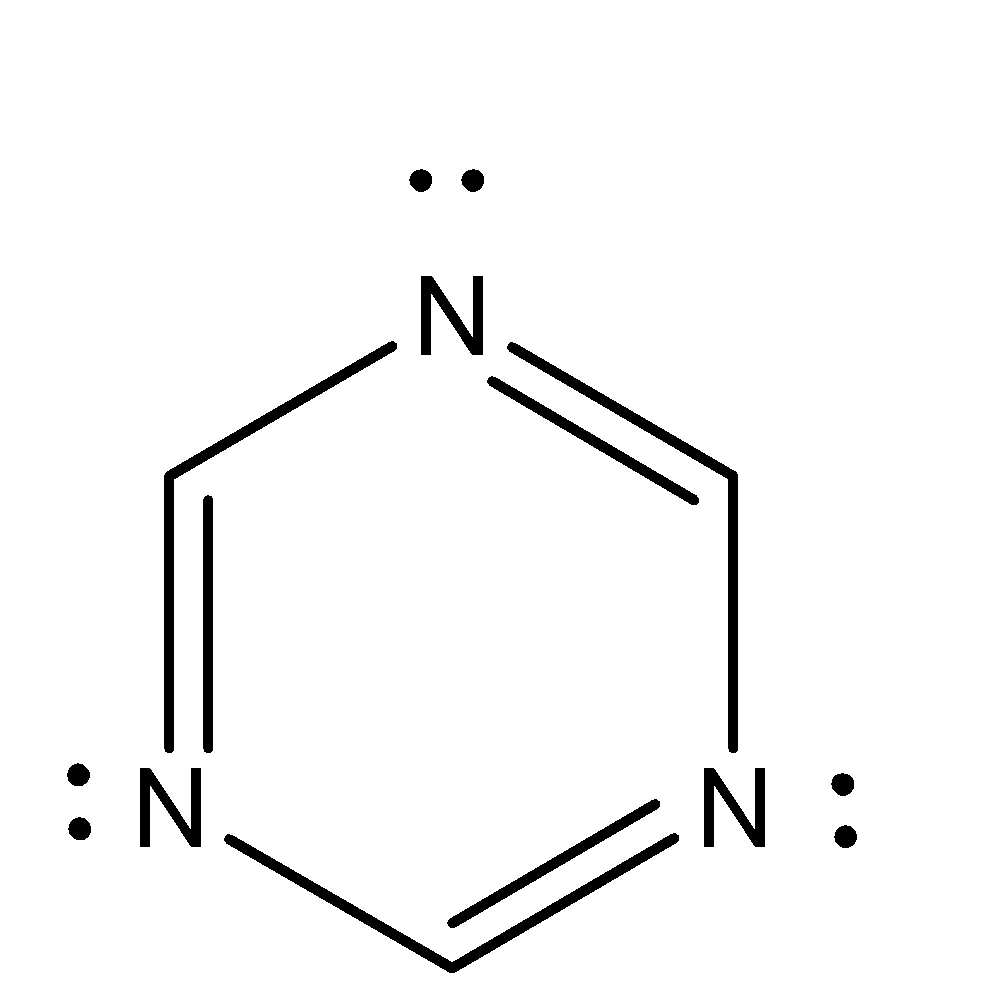
(i) ${C_3}H_3^{ + 1}$ (j)
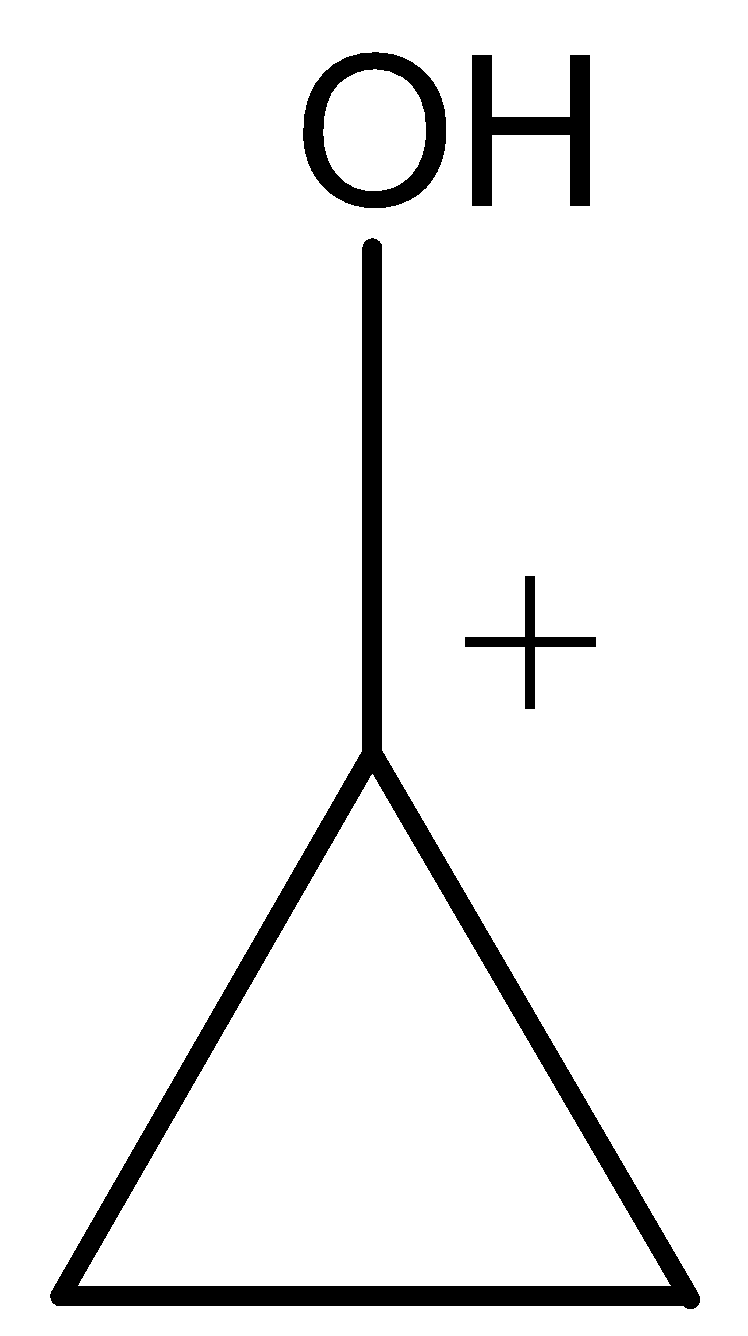
(k)
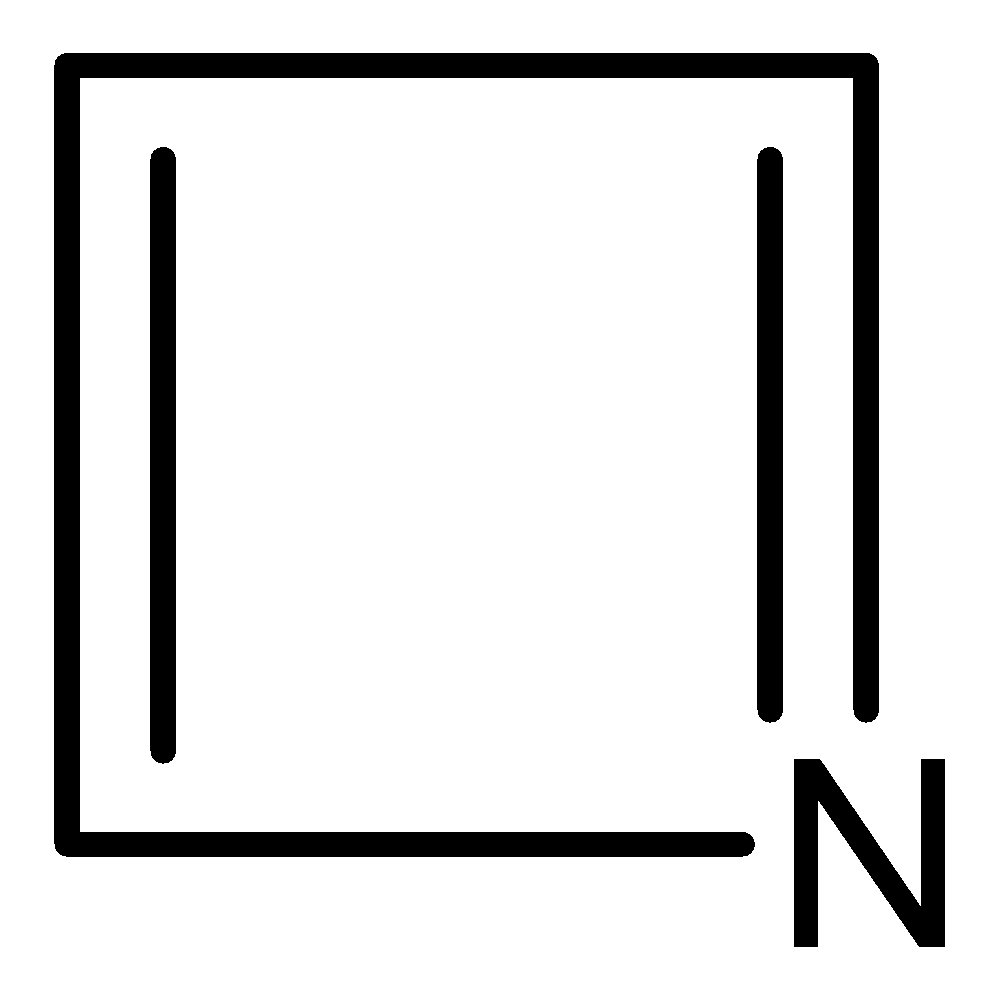
(l)
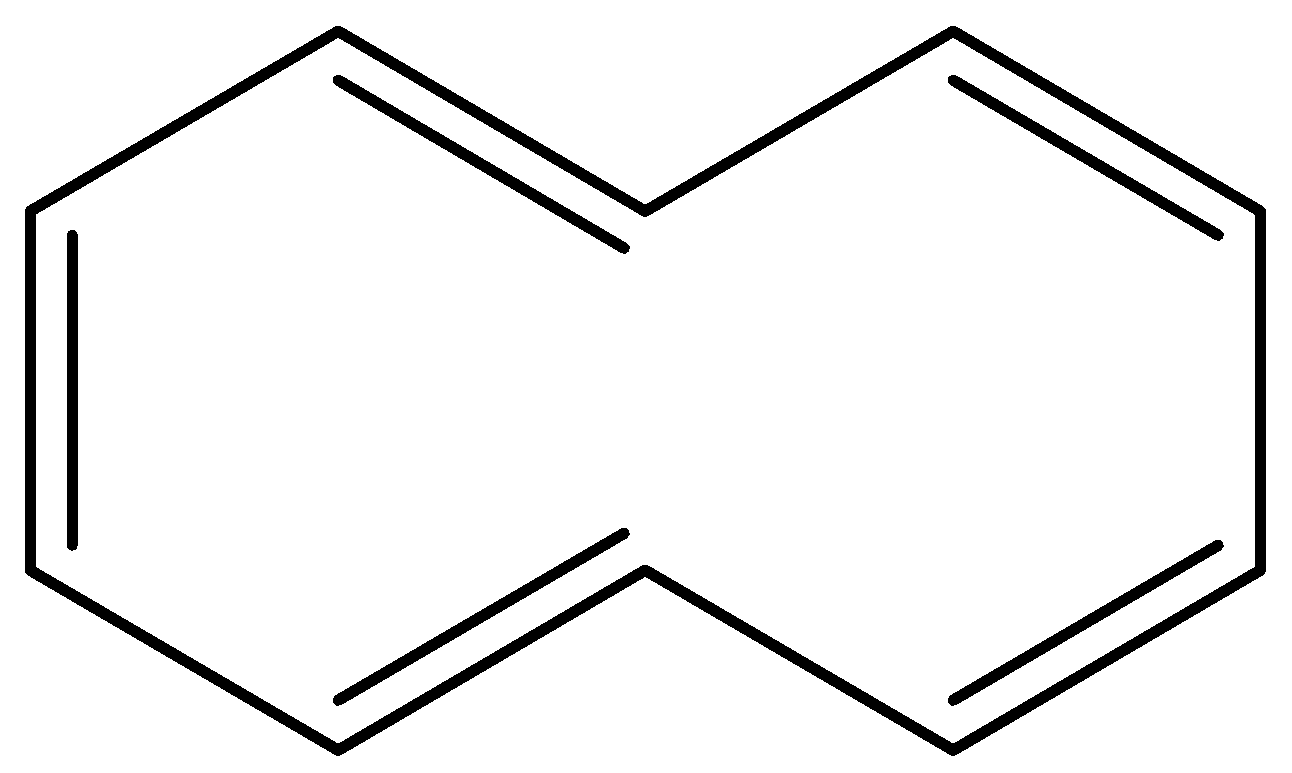
| Compound | Compound | Compound | |||
| (a) | 
| (b) | ${C_8}H_8^{ - 2}$ | (c) | 
|
| (d) | 
| (e) | 
| (f) | 
|
| (g) | 
| (h) | 
| (i) | ${C_3}H_3^{ + 1}$ |
| (j) | 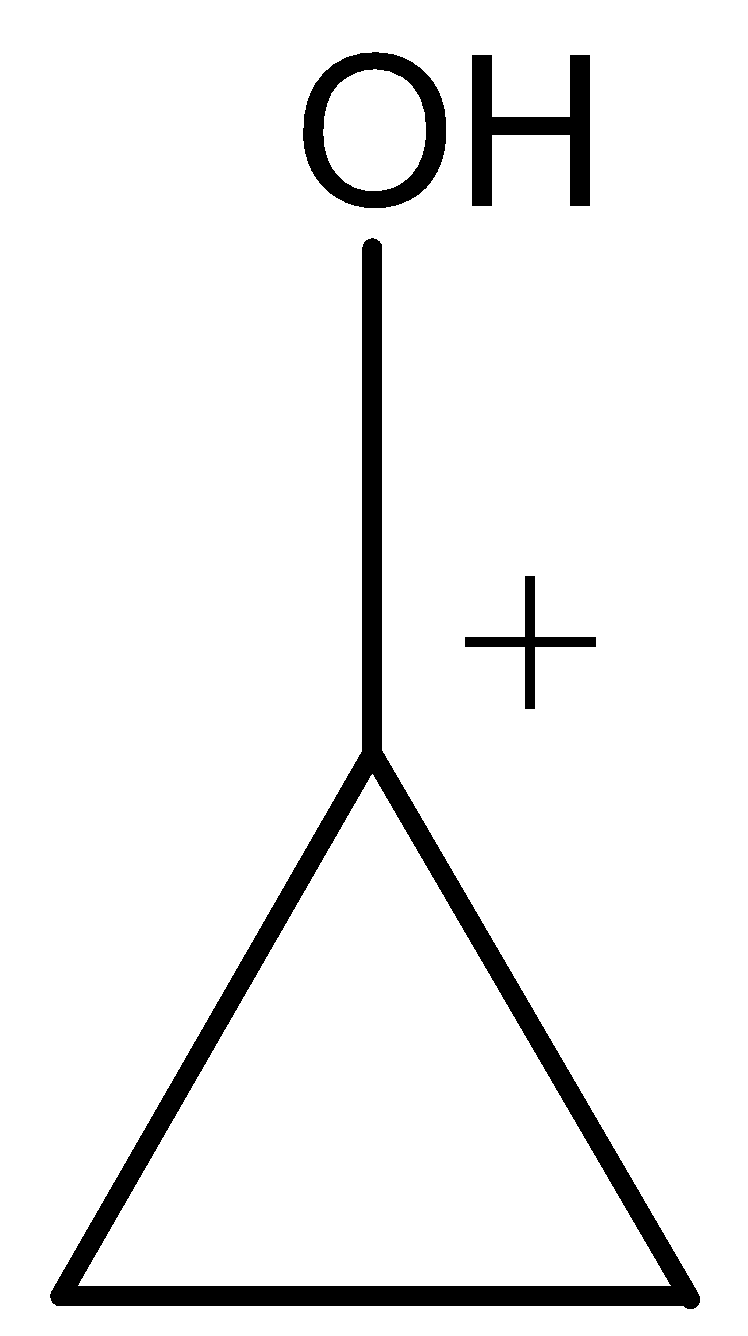
| (k) | 
| (l) | 
|
Answer
558k+ views
Hint: We must know that the dimerization happens only in anti-aromatic compounds, Aromatic are the rings with delocalized electron clouds, non-aromatics are opposite of aromatics. We need to know that benzene has a high degree of unsaturation, but it also has remarkable stability, and shows some unexpected behaviour. Those compounds having benzene type chemistry are conventionally known as the aromaticity of such compounds or aromatic character or aromaticity. Stability is due to delocalization of electrons present in $\pi - \pi $ orbitals.
Complete step by step answer:
Let’s start with discussing aromatic, non-aromatic, anti-aromatic and compounds that undergo dimerization.
Aromatic compounds are characterized by one or more planar rings of atoms joined by covalent bonds of two types.
Non-aromatic compounds are characterized either by non-ring structure called as aliphatic or those with ring are termed as alicyclic. Non-aromatic Compounds do not contain ring systems with delocalized electron clouds.
Anti-aromatic compounds are characterized by a high energy electron system due to the presence of 4n delocalized electrons.
Same molecular compounds which combine together to give adduct are known as dimerization compounds. Dimerization can happen only in anti-aromatic compounds.
We should need to know that if a compound does not contain benzene but shows aromatic characters then they are called non-benzenoid aromatic compounds. And also we have terms such as anti-aromatic compounds. Huckel was a German chemical physicist, he proposed a rule, states that if the number of $\pi $ electrons in the system is $(4n + 2)$ , the system is aromatic, and if it is $(4n)$ , that system is nonaromatic or antiaromatic.
Now, coming to the question
Aromatic compounds out of given compounds are compound (a), (b), (f), (h), (i), (j) Which means $w = 6$
Non-aromatic compounds out of given compounds are compound (d), (e), (g), (l) Which means $x = 4$
Anti-aromatic compounds out of given compounds are compound (c), (k) Which means $y = 2$
Only anti-aromatic undergoes dimerization which means $z = 2$
So, $w + x + y + z = 6 + 4 + 2 + 2 = 14$ .
Note: we are using many aromatic compounds in many industries. For example toluene is used as solvent and naphthalene is used as mothballs. These compounds are also used for manufacturing of drugs, dyes and explosives. Anti-aromatic compounds are unstable in nature and hence are not used directly as such in most of the cases.
Complete step by step answer:
Let’s start with discussing aromatic, non-aromatic, anti-aromatic and compounds that undergo dimerization.
Aromatic compounds are characterized by one or more planar rings of atoms joined by covalent bonds of two types.
Non-aromatic compounds are characterized either by non-ring structure called as aliphatic or those with ring are termed as alicyclic. Non-aromatic Compounds do not contain ring systems with delocalized electron clouds.
Anti-aromatic compounds are characterized by a high energy electron system due to the presence of 4n delocalized electrons.
Same molecular compounds which combine together to give adduct are known as dimerization compounds. Dimerization can happen only in anti-aromatic compounds.
We should need to know that if a compound does not contain benzene but shows aromatic characters then they are called non-benzenoid aromatic compounds. And also we have terms such as anti-aromatic compounds. Huckel was a German chemical physicist, he proposed a rule, states that if the number of $\pi $ electrons in the system is $(4n + 2)$ , the system is aromatic, and if it is $(4n)$ , that system is nonaromatic or antiaromatic.
Now, coming to the question
Aromatic compounds out of given compounds are compound (a), (b), (f), (h), (i), (j) Which means $w = 6$
Non-aromatic compounds out of given compounds are compound (d), (e), (g), (l) Which means $x = 4$
Anti-aromatic compounds out of given compounds are compound (c), (k) Which means $y = 2$
Only anti-aromatic undergoes dimerization which means $z = 2$
So, $w + x + y + z = 6 + 4 + 2 + 2 = 14$ .
Note: we are using many aromatic compounds in many industries. For example toluene is used as solvent and naphthalene is used as mothballs. These compounds are also used for manufacturing of drugs, dyes and explosives. Anti-aromatic compounds are unstable in nature and hence are not used directly as such in most of the cases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




