
What is the normal boiling point for chloroform?
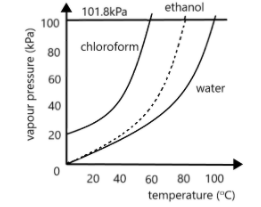
A. $40^\circ C$
B. $50^\circ C$
C. $60^\circ C$
D. $70^\circ C$
E. $80^\circ C$

Answer
569.4k+ views
Hint: The normal boiling point of a substance is the temperature at which boils at standard conditions of pressure and temperature. Thus, by drawing a vertical line from the point in the curve passing through the standard pressure downwards, we will get the temperature at which chloroform will boil.
Complete step by step answer:
Out of the three curves given, the first one corresponds to the boiling point curve of chloroform. As we can see there is a horizontal line passing through the graph at which the value of pressure is $101.8kPa$. As we know, this is the value of atmospheric pressure at standard room temperature conditions. Thus, the temperature corresponding to this point would give us the normal boiling point. For finding this, we draw a vertical line from the point where the chloroform curve meets this horizontal line downwards till it meets the $x$-axis. The intersection point on the $x$-axis will give us the normal boiling point. This is shown in the figure below:
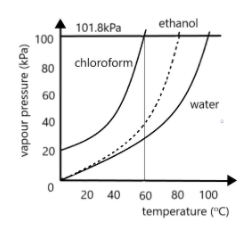
Thus, as we can see, this point corresponds approximately to the point $60$. Hence, the normal boiling point of chloroform is $60^\circ C$
So, the correct answer is Option C.
Note: The boiling point of a substance is the temperature at which its vapour pressure becomes equal to the external pressure. For raising the pressure of a constant volume system, we apply heat, till it reaches the boiling point. Note that chloroform is a highly volatile liquid used mainly as an organic solvent. It was earlier used as an anaesthetic.
Complete step by step answer:
Out of the three curves given, the first one corresponds to the boiling point curve of chloroform. As we can see there is a horizontal line passing through the graph at which the value of pressure is $101.8kPa$. As we know, this is the value of atmospheric pressure at standard room temperature conditions. Thus, the temperature corresponding to this point would give us the normal boiling point. For finding this, we draw a vertical line from the point where the chloroform curve meets this horizontal line downwards till it meets the $x$-axis. The intersection point on the $x$-axis will give us the normal boiling point. This is shown in the figure below:

Thus, as we can see, this point corresponds approximately to the point $60$. Hence, the normal boiling point of chloroform is $60^\circ C$
So, the correct answer is Option C.
Note: The boiling point of a substance is the temperature at which its vapour pressure becomes equal to the external pressure. For raising the pressure of a constant volume system, we apply heat, till it reaches the boiling point. Note that chloroform is a highly volatile liquid used mainly as an organic solvent. It was earlier used as an anaesthetic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




