
How is Nitronium ion formed from a nitrating mixture?
Answer
512.1k+ views
Hint: Formation of nitronium ions can be easily tracked when we look at the mechanism of nitration reaction’s mechanism. Also, we should know what a nitrating mixture is and what are the chemical compounds that form the nitrating mixture. Those compounds are nitric acid and sulphuric acid.
Complete answer:
The formation of nitronium takes place in many reactions which have nitrating mixture present in them. Nitrating mixture is a combination of concentrated sulphuric acid and nitric acid $({H_2}S{O_4} + HN{O_3})$. The most common type of reaction where we see the generation of nitronium ions is electrophilic substitution reaction.
Nitronium ion is a cation with the molecular formula $N{O_2}^ + $, in which the positive charge resides on the nitrogen atom. The structure of nitronium ion is given below:
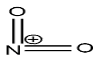
now, about the explanation of generation of nitronium ion from the nitrating mixture is given with the help the following mechanism:
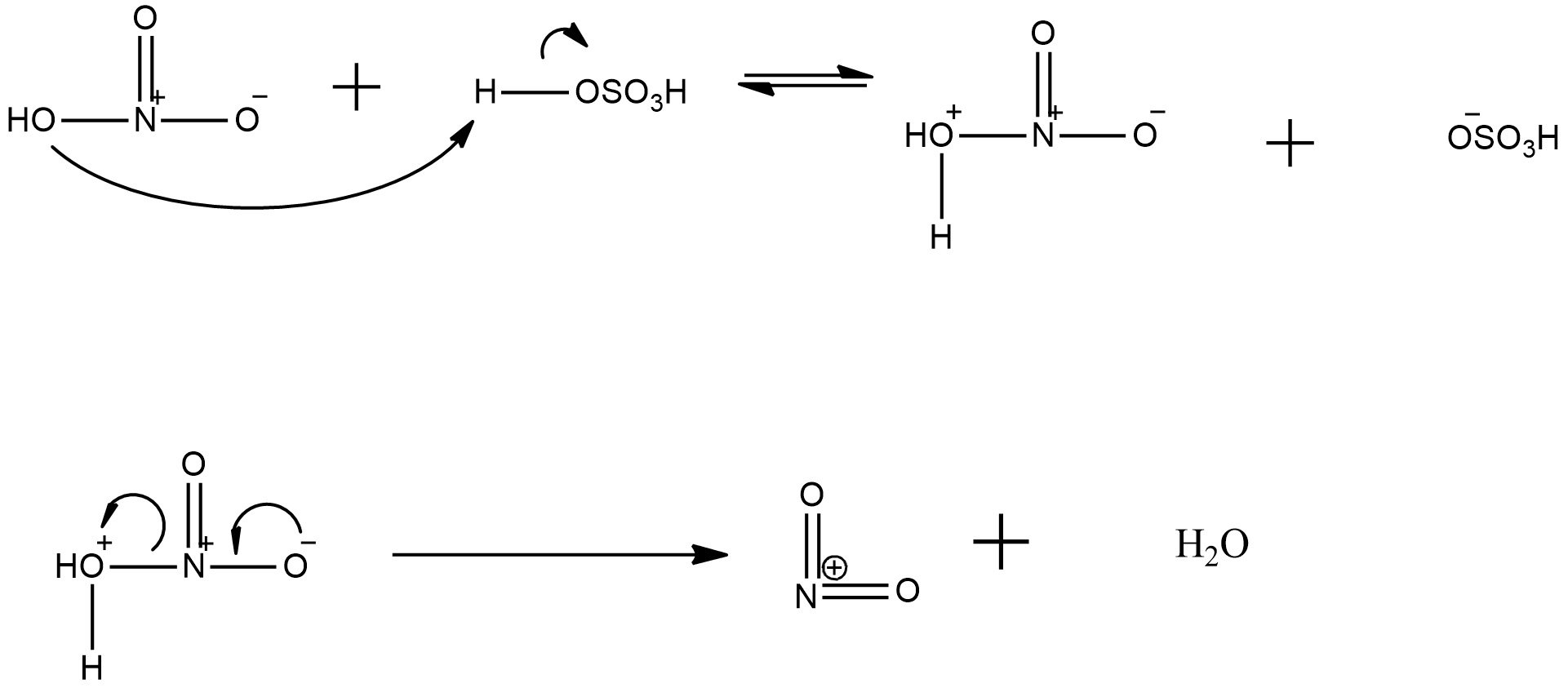
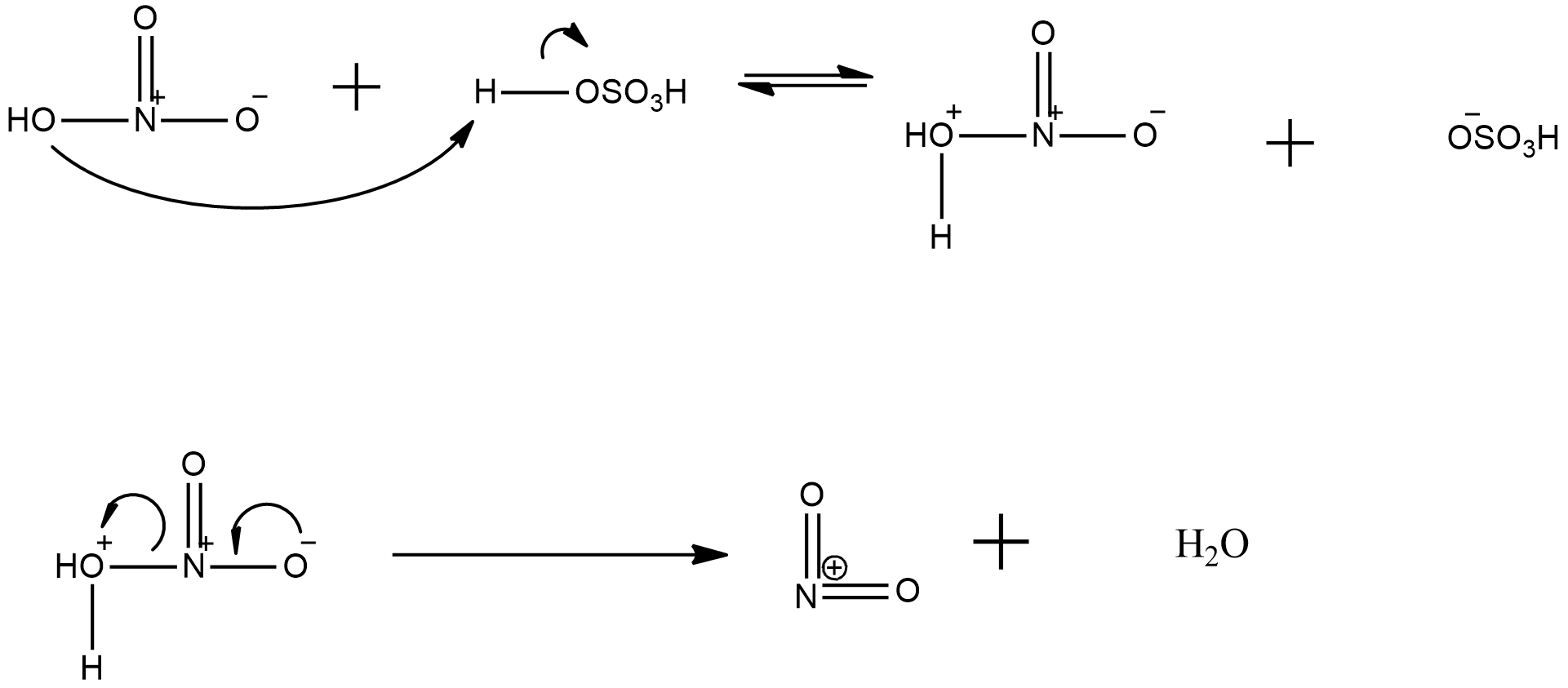
Hence, it can be seen clearly that in nitronium ion formation needs a proton donor, such as sulphuric acid and then there is release of a water molecule and the nitronium ion is formed.
Note:
In the nitronium ion formation, although we need sulphuric acid in the start, but eventually as the reaction continues, the anion of sulphuric acid that is formed in the start, it again gains the proton from the further products that are formed in the reaction and converts back to sulphuric acid. Common example where we use nitronium ion is conversion of benzene to nitrobenzene.
Complete answer:
The formation of nitronium takes place in many reactions which have nitrating mixture present in them. Nitrating mixture is a combination of concentrated sulphuric acid and nitric acid $({H_2}S{O_4} + HN{O_3})$. The most common type of reaction where we see the generation of nitronium ions is electrophilic substitution reaction.
Nitronium ion is a cation with the molecular formula $N{O_2}^ + $, in which the positive charge resides on the nitrogen atom. The structure of nitronium ion is given below:
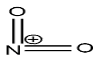
now, about the explanation of generation of nitronium ion from the nitrating mixture is given with the help the following mechanism:


Hence, it can be seen clearly that in nitronium ion formation needs a proton donor, such as sulphuric acid and then there is release of a water molecule and the nitronium ion is formed.
Note:
In the nitronium ion formation, although we need sulphuric acid in the start, but eventually as the reaction continues, the anion of sulphuric acid that is formed in the start, it again gains the proton from the further products that are formed in the reaction and converts back to sulphuric acid. Common example where we use nitronium ion is conversion of benzene to nitrobenzene.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




