
[NiCl$_4$]$^{2-}$ is paramagnetic while [Ni(CO)$_4$] diamagnetic though both are tetrahedral. Why? (Atomic No. of Ni = 28)
Answer
596.1k+ views
Hint: We can determine the reason for different magnetic nature on the basis of the nature of ligands attached to the nickel. Secondly, we can identify on the basis of electronic configuration, and the oxidation state of nickel. We know that the complex having unpaired electrons is paramagnetic, whereas the complex having paired electrons is diamagnetic. So, the reason would be known.
Complete step by step solution:
First, we will determine the reason on the basis of the nature of ligands attached to it.
Now, we have two coordination complex of nickel, i.e. [NiCl$_4$]$^{2-}$, and [Ni(CO)$_4$]. In the first one, there is a chlorine ligand attached to the nickel, and in the second one, a carbonyl ligand is attached.
Thus, we will calculate the oxidation state of nickel in the complexes given.
First we have [NiCl$_4$]$^{2-}$
Here oxidation state of nickel will be x + 4 (-1) = -2,
We get x = +2.
Second we have [Ni(CO)$_4$]
Here x + 4(0) = 0,
We get x = 0.
We can say that in the first one nickel has +2, and in the second one it has 0 oxidation state respectively.
Talking about the nature of chlorine ligand; it is considered to be the weak field ligand. As we know, the weak field ligands don’t show the pairing of unpaired electrons.
So, we can say that the first complex is paramagnetic.
Now, if we talk about the second one, we know that nickel exists in 0 oxidation state as mentioned. The ligand attached to nickel is carbonyl (CO); i.e. a strong field ligand.
We know that the strong field causes pairing of unpaired electrons. Thus, there will be no unpaired electrons in the second case.
So, we can say that the second complex is diamagnetic.
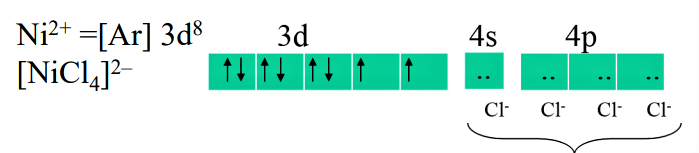
We can represent the nickel in first case i.e. [NiCl$_4$]$^{2-}$
In the second case i.e. [Ni(CO)$_4$], we have Ni = [Ar] 3d$^{8}$4s$^{2}$. Thus, the two electrons will be placed in the 4s orbital.
In the last, we can conclude that in [Ni(CO)$_4$] due to the pairing of electrons; it is paramagnetic; whereas in [NiCl$_4$]$^{2-}$, due to the unpaired electrons; it is paramagnetic.
Note: Don’t get confused while determining the reason for the magnetic nature of the complexes. It is all based on the Hund’s rule for multiplicity. Just move step by step to avoid the confusion. Also, The weak field and strong field ligand can be identified through spectrochemical series. Ligands that produce large splitting are called strong field ligands and which produce little splitting are called weak field ligands.
Complete step by step solution:
First, we will determine the reason on the basis of the nature of ligands attached to it.
Now, we have two coordination complex of nickel, i.e. [NiCl$_4$]$^{2-}$, and [Ni(CO)$_4$]. In the first one, there is a chlorine ligand attached to the nickel, and in the second one, a carbonyl ligand is attached.
Thus, we will calculate the oxidation state of nickel in the complexes given.
First we have [NiCl$_4$]$^{2-}$
Here oxidation state of nickel will be x + 4 (-1) = -2,
We get x = +2.
Second we have [Ni(CO)$_4$]
Here x + 4(0) = 0,
We get x = 0.
We can say that in the first one nickel has +2, and in the second one it has 0 oxidation state respectively.
Talking about the nature of chlorine ligand; it is considered to be the weak field ligand. As we know, the weak field ligands don’t show the pairing of unpaired electrons.
So, we can say that the first complex is paramagnetic.
Now, if we talk about the second one, we know that nickel exists in 0 oxidation state as mentioned. The ligand attached to nickel is carbonyl (CO); i.e. a strong field ligand.
We know that the strong field causes pairing of unpaired electrons. Thus, there will be no unpaired electrons in the second case.
So, we can say that the second complex is diamagnetic.
We can represent the nickel in first case i.e. [NiCl$_4$]$^{2-}$

In the second case i.e. [Ni(CO)$_4$], we have Ni = [Ar] 3d$^{8}$4s$^{2}$. Thus, the two electrons will be placed in the 4s orbital.
In the last, we can conclude that in [Ni(CO)$_4$] due to the pairing of electrons; it is paramagnetic; whereas in [NiCl$_4$]$^{2-}$, due to the unpaired electrons; it is paramagnetic.
Note: Don’t get confused while determining the reason for the magnetic nature of the complexes. It is all based on the Hund’s rule for multiplicity. Just move step by step to avoid the confusion. Also, The weak field and strong field ligand can be identified through spectrochemical series. Ligands that produce large splitting are called strong field ligands and which produce little splitting are called weak field ligands.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




