
Neopentyl chloride on reaction with ethanolic KOH is likely to:
(a)Neopentyl alcohol
(b)pentylene
(c)2-methyl-2-butene
(d)undergo no reaction
Answer
592.5k+ views
Hint: Haloalkanes can undergo elimination reaction in the presence of concentrated solution of sodium or potassium hydroxide in ethanol. It is usually heated under reflux.
Complete step by step solution:
Elimination or dehydrohalogenation occurs in haloalkanes in the presence of ethanolic KOH or alcoholic KOH.
Alcoholic KOH, especially in ethanol, produce \[{{C}_{2}}{{H}_{5}}{{O}_{{}}}-\]ions. These ions are much stronger than the base \[O{{H}^{-}}\]ion. \[{{C}_{2}}{{H}_{5}}{{O}_{{}}}-\]ion has the capability to abstract \[\beta \]hydrogen of the alkyl halide and can convert haloalkane to an alkene.
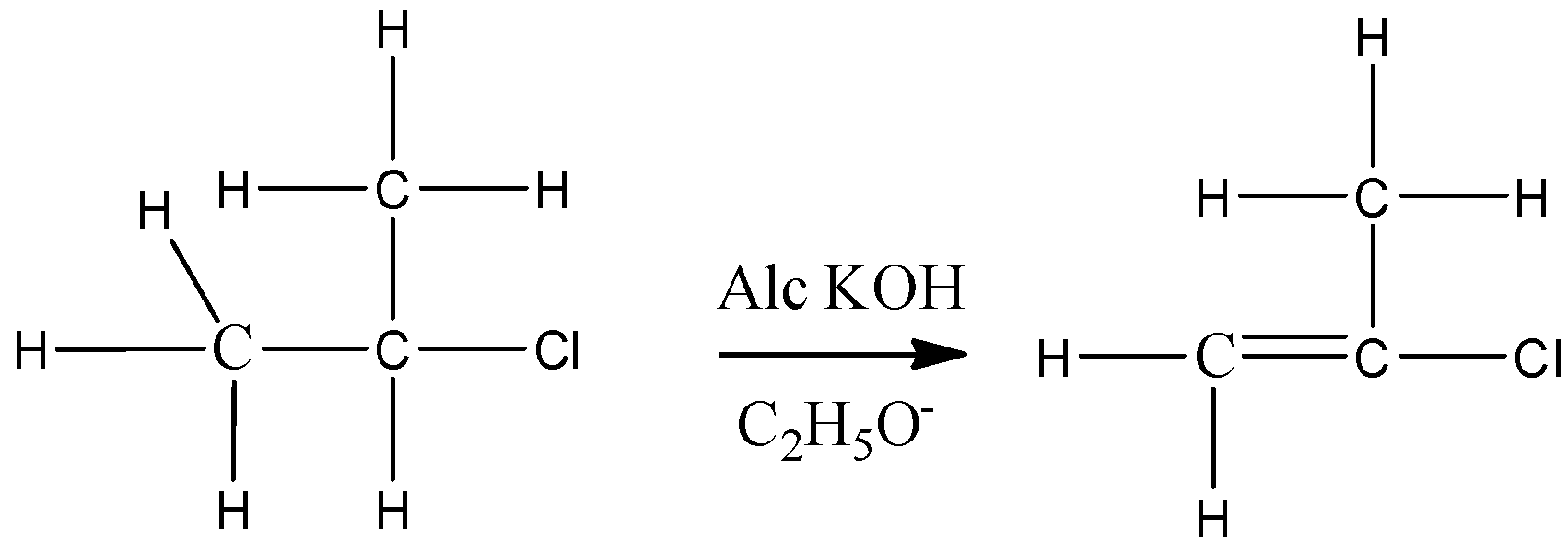
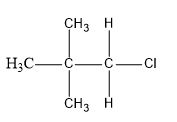
For elimination reactions to occur, there must be a \[\beta \]hydrogen in the alkyl halide, then only it can abstract this hydrogen and form a double bond. In case of neopentyl there is no \[\beta \]hydrogen.
Therefore, it cannot undergo elimination reaction in the presence of ethanolic KOH.
The correct answer to the question is option (d).
Additional Information: This elimination reaction is also used for the preparation of alkenes. Alkenes of desired chain length can be prepared too and it follows Saytzeff's rule. The ease of dehydrohalogenation of different types of haloalkanes follows the order\[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}\]. This can be explained using saytzeff’s rule. The alkene with greater number of alkyl groups at doubly bonded carbon atoms is a preferred product. This implies that more substituted alkene is more stable and it is formed faster.
Note: We should always keep in mind that, while an elimination reaction occurs in a haloalkane, there must be a\[\beta \]hydrogen, otherwise the reaction will not happen.( Beta carbon atom is the carbon atom which is placed in second position from a functional group and the hydrogen directly bonded to beta carbon is called beta hydrogen). Because aldol condensation reaction is driven by the presence of beta hydrogen.
Complete step by step solution:
Elimination or dehydrohalogenation occurs in haloalkanes in the presence of ethanolic KOH or alcoholic KOH.
Alcoholic KOH, especially in ethanol, produce \[{{C}_{2}}{{H}_{5}}{{O}_{{}}}-\]ions. These ions are much stronger than the base \[O{{H}^{-}}\]ion. \[{{C}_{2}}{{H}_{5}}{{O}_{{}}}-\]ion has the capability to abstract \[\beta \]hydrogen of the alkyl halide and can convert haloalkane to an alkene.

For elimination reactions to occur, there must be a \[\beta \]hydrogen in the alkyl halide, then only it can abstract this hydrogen and form a double bond. In case of neopentyl there is no \[\beta \]hydrogen.

Therefore, it cannot undergo elimination reaction in the presence of ethanolic KOH.
The correct answer to the question is option (d).
Additional Information: This elimination reaction is also used for the preparation of alkenes. Alkenes of desired chain length can be prepared too and it follows Saytzeff's rule. The ease of dehydrohalogenation of different types of haloalkanes follows the order\[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}\]. This can be explained using saytzeff’s rule. The alkene with greater number of alkyl groups at doubly bonded carbon atoms is a preferred product. This implies that more substituted alkene is more stable and it is formed faster.
Note: We should always keep in mind that, while an elimination reaction occurs in a haloalkane, there must be a\[\beta \]hydrogen, otherwise the reaction will not happen.( Beta carbon atom is the carbon atom which is placed in second position from a functional group and the hydrogen directly bonded to beta carbon is called beta hydrogen). Because aldol condensation reaction is driven by the presence of beta hydrogen.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




