
Name three fundamental particles of the atom. Give the symbol with charge, on each particle.
Answer
566.7k+ views
Hint: Atom is the fundamental building block of a matter. It is the smallest unit of the matter which forms a chemical element. Atom itself is composed of many subatomic particles. Many atoms will combine together to form a molecule.
Complete Solution :
- Let’s now understand about atoms. What is an atom? Atoms are the building block of the matter. Many atoms combine to form a molecule. Various solid, liquid and gases are composed of the atoms. Atom is made up of many lightest particles. These particles are called subatomic particles. The subatomic particles of an atom are proton, neutron, electrons, alpha particles and beta particles. Among these protons, neutrons and electrons are the three fundamental particles of atoms.
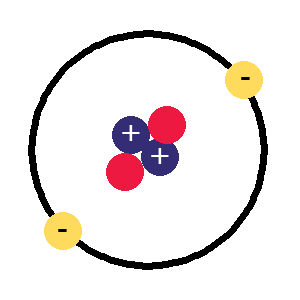
- Atoms are made up of a nucleus with the electron revolving around it. Nucleus is a small dense material which is composed of nucleons. Protons and neutrons together are called nucleons. Atom’s mass is present in the nucleus. In the atom, electrons revolving around the nucleus will have a negative charge. The proton present in the nucleus is said to have a positive charge and neutrons will have a neutral charge.
Other than fundamental particles, atoms can also have other subatomic particles. They are called alpha and beta-particles.
- Alpha Particles:
Particles that are emitted by \[\alpha - decay\] are called as \[\alpha - particles\]. Alpha particles are denoted by \[\alpha \] or \[H{e^{2 + }}\] \[\alpha - \] decay can take place only when the element is a radioactive element.
- Beta particles:
Particles which are emitted during \[\beta - decay\] are called \[\beta - particles\]. \[\beta - particles\] are denoted by \[\beta \] .
Note: We know that Dalton was the one who first proposed the atomic model. In his findings he said that an atom is a tiny, indivisible particle. Later it was J.J Thomson who said that atoms can be divided into sub-atomic particles by discovering electrons. Further other fundamental particles were also discovered by other scientists.
Complete Solution :
- Let’s now understand about atoms. What is an atom? Atoms are the building block of the matter. Many atoms combine to form a molecule. Various solid, liquid and gases are composed of the atoms. Atom is made up of many lightest particles. These particles are called subatomic particles. The subatomic particles of an atom are proton, neutron, electrons, alpha particles and beta particles. Among these protons, neutrons and electrons are the three fundamental particles of atoms.

- Atoms are made up of a nucleus with the electron revolving around it. Nucleus is a small dense material which is composed of nucleons. Protons and neutrons together are called nucleons. Atom’s mass is present in the nucleus. In the atom, electrons revolving around the nucleus will have a negative charge. The proton present in the nucleus is said to have a positive charge and neutrons will have a neutral charge.
| Particles | Charge | Symbol | Atomic charge |
| Proton | \[ + 1.6022 \times {10^{ - 19}}\] | \[{p^ + }\] | +1 |
| Neutron | 0 | \[{n^0}\] | 0 |
| Electron | \[ - 1.6022 \times {10^{ - 19}}\] | \[{e^ - }\] | -1 |
Other than fundamental particles, atoms can also have other subatomic particles. They are called alpha and beta-particles.
- Alpha Particles:
Particles that are emitted by \[\alpha - decay\] are called as \[\alpha - particles\]. Alpha particles are denoted by \[\alpha \] or \[H{e^{2 + }}\] \[\alpha - \] decay can take place only when the element is a radioactive element.
- Beta particles:
Particles which are emitted during \[\beta - decay\] are called \[\beta - particles\]. \[\beta - particles\] are denoted by \[\beta \] .
Note: We know that Dalton was the one who first proposed the atomic model. In his findings he said that an atom is a tiny, indivisible particle. Later it was J.J Thomson who said that atoms can be divided into sub-atomic particles by discovering electrons. Further other fundamental particles were also discovered by other scientists.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




