
Name the compound of chlorine
A.Which is an anesthetic.
B.Which is used as a refrigerant.
C.Which is a disinfectant.
D.Which is used to make pipes and tubes.
E.Which liberates chlorine on exposure to air.
Answer
572.1k+ views
Hint:The compound known as trichloromethane is a colorless organic compound having the chemical formula as $CHC{l_3}$ with strong-smelling liquid. It is a very powerful anesthetic.
Chlorofluorocarbon consists of carbon, hydrogen, chlorine, and fluorine and is categorized under halocarbon compounds. This compound is widely used as aerosol propellants and refrigerants.
The chemical name of a bleaching agent is calcium hypochlorite. Various bleach chemicals are oxidizing agents.
Complete step by step answer:
1.Chloroform:
-Chloroform or trichloromethane is an organic compound.
-The chlorine compound which is used as an anesthetic and sedative is chloroform. Anesthetic is a drug by which a person does not feel pain during surgery or operation.
-The chemical formula of chloroform is $CHC{l_3}$ and it is colorless. It is very less used as an anesthetic because its effects on the body can be serious, resulting in damage to the liver and the kidneys.
2.Chlorofluorocarbons (CFC):
Chlorofluorocarbons are a compound of chlorine which is used as a refrigerant. A refrigerant is a substance used in the refrigerant.
-Their boiling points are close to zero degrees centigrade, and they represent the property volatility.
-The physical properties ideal for use as refrigerant gases in air conditioners, freezers, and refrigerators.
-CFC has some harmful effects as it leads to global warming and ozone layer depletion in the environment.
3.Sodium hypochlorite (NaOCl) and Calcium hypochlorite:
-Sodium hypochlorite and calcium hypochlorite are used as a disinfectant. Disinfectant is a chemical that kills the bacteria or microorganisms.
-Sodium hypochlorite is a yellowish liquid and uses sodium hypochlorite. Disinfection is achieved at low concentrations of chlorine in water and sodium does not affect water. Calcium hypochlorite can be found as white powder or in the form of tablets so it can be dissolved in warm water.
-Sodium hypochlorite is most known as a pale greenish-yellow dilute solution referred to as liquid bleach, which is a household chemical widely used as a disinfectant or a bleaching agent.
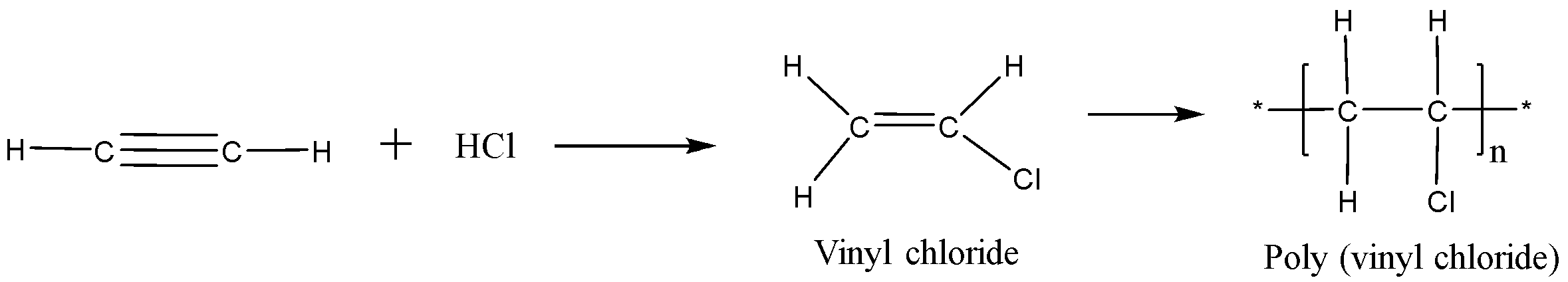
4.PVC (polyvinyl chloride) is a chlorine compound used to make pipes and tubes. Chlorine and ethylene gas react together to form vinyl chloride at high temperature then there is polymerization of vinyl chloride and it forms PVC. In polymerization small molecules or monomer units like (vinyl chloride) join together to make very long molecules like PVC.
5.Bleaching powder liberates chlorine on exposure to air. Its chemical formula is calcium oxy-chloride ($CaOC{l_2}$) and it is a yellowish powder. When bleaching powder is exposed to air, it reacts with the surrounding carbon dioxide to form chlorine gas and calcium carbonate. Its chemical equation is:
$CaOC{l_2} + C{O_2} \to CaC{O_3} + C{l_2}$
Note:
Chloroform is often formed as a by-product in the process of water chlorination along with a range of other disinfection by-products and thus is present in municipal tap water and swimming pools.
Chlorofluorocarbons cause depletion of the atmospheric ozone layer.
Bleaching powder after mixing with the dishwasher in water is used to clean old items.
Chlorofluorocarbon consists of carbon, hydrogen, chlorine, and fluorine and is categorized under halocarbon compounds. This compound is widely used as aerosol propellants and refrigerants.
The chemical name of a bleaching agent is calcium hypochlorite. Various bleach chemicals are oxidizing agents.
Complete step by step answer:
1.Chloroform:
-Chloroform or trichloromethane is an organic compound.
-The chlorine compound which is used as an anesthetic and sedative is chloroform. Anesthetic is a drug by which a person does not feel pain during surgery or operation.
-The chemical formula of chloroform is $CHC{l_3}$ and it is colorless. It is very less used as an anesthetic because its effects on the body can be serious, resulting in damage to the liver and the kidneys.
2.Chlorofluorocarbons (CFC):
Chlorofluorocarbons are a compound of chlorine which is used as a refrigerant. A refrigerant is a substance used in the refrigerant.
-Their boiling points are close to zero degrees centigrade, and they represent the property volatility.
-The physical properties ideal for use as refrigerant gases in air conditioners, freezers, and refrigerators.
-CFC has some harmful effects as it leads to global warming and ozone layer depletion in the environment.
3.Sodium hypochlorite (NaOCl) and Calcium hypochlorite:
-Sodium hypochlorite and calcium hypochlorite are used as a disinfectant. Disinfectant is a chemical that kills the bacteria or microorganisms.
-Sodium hypochlorite is a yellowish liquid and uses sodium hypochlorite. Disinfection is achieved at low concentrations of chlorine in water and sodium does not affect water. Calcium hypochlorite can be found as white powder or in the form of tablets so it can be dissolved in warm water.
-Sodium hypochlorite is most known as a pale greenish-yellow dilute solution referred to as liquid bleach, which is a household chemical widely used as a disinfectant or a bleaching agent.
4.PVC (polyvinyl chloride) is a chlorine compound used to make pipes and tubes. Chlorine and ethylene gas react together to form vinyl chloride at high temperature then there is polymerization of vinyl chloride and it forms PVC. In polymerization small molecules or monomer units like (vinyl chloride) join together to make very long molecules like PVC.

5.Bleaching powder liberates chlorine on exposure to air. Its chemical formula is calcium oxy-chloride ($CaOC{l_2}$) and it is a yellowish powder. When bleaching powder is exposed to air, it reacts with the surrounding carbon dioxide to form chlorine gas and calcium carbonate. Its chemical equation is:
$CaOC{l_2} + C{O_2} \to CaC{O_3} + C{l_2}$
Note:
Chloroform is often formed as a by-product in the process of water chlorination along with a range of other disinfection by-products and thus is present in municipal tap water and swimming pools.
Chlorofluorocarbons cause depletion of the atmospheric ozone layer.
Bleaching powder after mixing with the dishwasher in water is used to clean old items.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




