
Name the catalyst used in the commercial method of preparation of phenol.
(A) Silica
(B) Calcium phosphate
(C) Anhydrous aluminium chloride
(D) Cobalt naphthenate
Answer
524.7k+ views
Hint: As we know that catalyst is a substance which enables a chemical reaction to proceed at a faster rate or maybe under different conditions than otherwise possible such as at a lower temperature. These chemicals are not consumed during the catalyzed reaction but can act repeatedly. So here we have to talk about the catalyst which is used in the preparation of phenol.
Complete answer:
Let us discuss about catalyst as follows:-
Catalyst: These are the substances which enable a chemical reaction to proceed at a faster rate or maybe under different conditions than otherwise possible such as at a lower temperature. Generally very small amounts of catalyst are required during a chemical reaction. These chemicals are not consumed during the catalyzed reaction but can act repeatedly.
-Preparation of phenol: Phenol is prepared by various methods. One of the methods is aerial oxidation of cumene in the presence of a catalyst. The synthesis is shown below:-
(a) Use of Cobalt naphthenate:-

Cumene which is also known as isopropyl benzene is first oxidized in the presence of air to form cumene hydroperoxide. This is done with the help of Cobalt naphthenate at alkaline medium and at a temperature of 473 Kelvin.
-Reaction of cumene hydroperoxide with dilute sulphuric acid:-
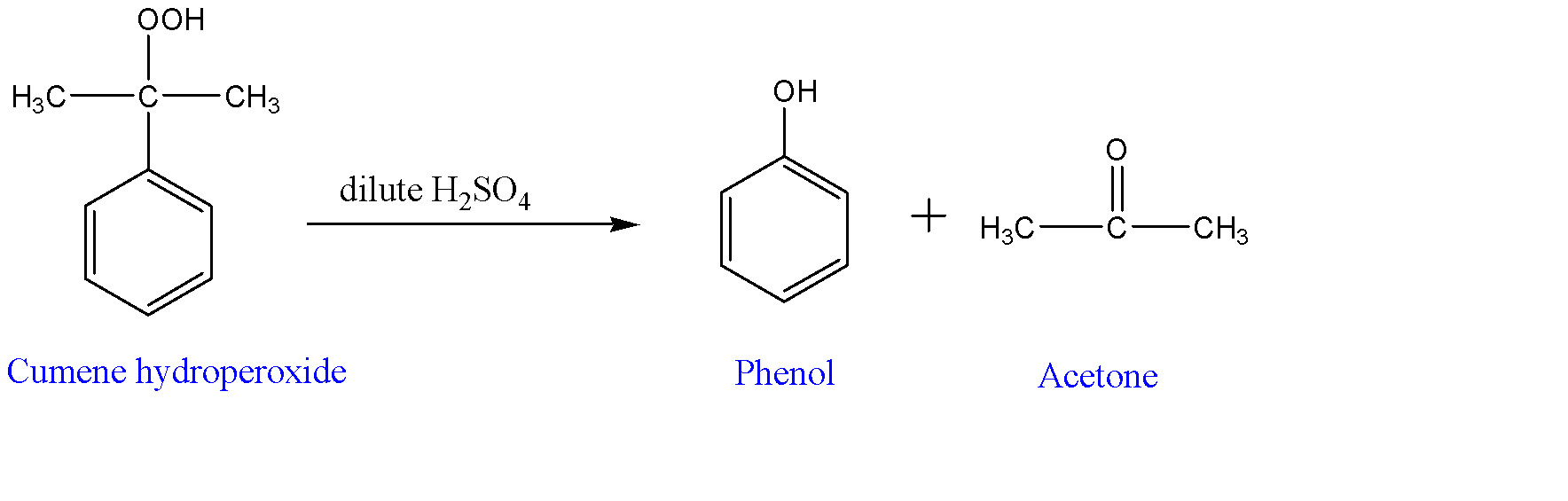
Cumene hydroperoxide is now treated with dilute sulphuric acid and this is performed along with the supply of heat. It yields phenol along with acetone as by-products.
-Therefore the catalyst used in the commercial method of preparation of phenol is (D) Cobalt naphthenate.
Note:
-Remember that this synthesis of phenol from cumene yields acetone in very large amounts. Therefore phenol prepared by this method requires purification as well.
-Also cumene is generally obtained by Friedel-Crafts alkylation of benzene with propylene.
Complete answer:
Let us discuss about catalyst as follows:-
Catalyst: These are the substances which enable a chemical reaction to proceed at a faster rate or maybe under different conditions than otherwise possible such as at a lower temperature. Generally very small amounts of catalyst are required during a chemical reaction. These chemicals are not consumed during the catalyzed reaction but can act repeatedly.
-Preparation of phenol: Phenol is prepared by various methods. One of the methods is aerial oxidation of cumene in the presence of a catalyst. The synthesis is shown below:-
(a) Use of Cobalt naphthenate:-

Cumene which is also known as isopropyl benzene is first oxidized in the presence of air to form cumene hydroperoxide. This is done with the help of Cobalt naphthenate at alkaline medium and at a temperature of 473 Kelvin.
-Reaction of cumene hydroperoxide with dilute sulphuric acid:-

Cumene hydroperoxide is now treated with dilute sulphuric acid and this is performed along with the supply of heat. It yields phenol along with acetone as by-products.
-Therefore the catalyst used in the commercial method of preparation of phenol is (D) Cobalt naphthenate.
Note:
-Remember that this synthesis of phenol from cumene yields acetone in very large amounts. Therefore phenol prepared by this method requires purification as well.
-Also cumene is generally obtained by Friedel-Crafts alkylation of benzene with propylene.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




