
What is the name of the given structure?
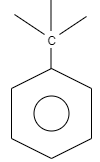
A. Benzo
B. Benzal
C. Benzyl
D. None of the above

Answer
557.7k+ views
Hint:Chemistry is a study of different compounds and elements that have different physical properties and chemical properties. Every compound has a molecular formula on the basis of which a structure of compound is formed. Chemical formula is used to represent any chemical substance with the help of symbols of atoms present in it.
Complete step-by-step answer: Let us discuss the structure of above compounds one by one:
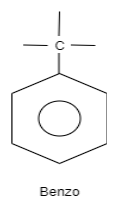
A. Benzo is an organic structure which is classified as an aromatic hydrocarbon. The structure of benzo consists of a benzene ring which is attached to carbon which is further attached to carbon substituted groups. These substituted groups can alkyl groups, phenyl groups so on.
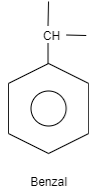
B. Benzal is an organic structure which is classified as an aromatic hydrocarbon. The structure of benzal consists of a benzene ring which is attached to carbon which is further attached to carbon having two substituted groups and one hydrogen. These substituted groups can be alkyl groups, phenyl groups and so on.
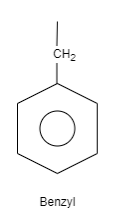
C. Benzyl is an organic structure which is classified as an aromatic hydrocarbon. The structure of benzyl consists of a benzene ring which is attached to carbon which is further attached to carbon having one substituted group and two hydrogen atoms. These substituted groups can be alkyl groups, phenyl groups and so on.
Therefore, the correct option is A.
Note:It is to note that benzene is a hydrocarbon. There are many derivatives of benzene which consist of substituted compounds. The above options are the examples of benzene substituted compounds. Benzene compounds consist of single substituted compounds, di substituted compounds and poly substituted compounds.
Complete step-by-step answer: Let us discuss the structure of above compounds one by one:
A. Benzo is an organic structure which is classified as an aromatic hydrocarbon. The structure of benzo consists of a benzene ring which is attached to carbon which is further attached to carbon substituted groups. These substituted groups can alkyl groups, phenyl groups so on.

B. Benzal is an organic structure which is classified as an aromatic hydrocarbon. The structure of benzal consists of a benzene ring which is attached to carbon which is further attached to carbon having two substituted groups and one hydrogen. These substituted groups can be alkyl groups, phenyl groups and so on.

C. Benzyl is an organic structure which is classified as an aromatic hydrocarbon. The structure of benzyl consists of a benzene ring which is attached to carbon which is further attached to carbon having one substituted group and two hydrogen atoms. These substituted groups can be alkyl groups, phenyl groups and so on.

Therefore, the correct option is A.
Note:It is to note that benzene is a hydrocarbon. There are many derivatives of benzene which consist of substituted compounds. The above options are the examples of benzene substituted compounds. Benzene compounds consist of single substituted compounds, di substituted compounds and poly substituted compounds.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




