
What is the name of $ N{O^{3 - }} $ ?
The information I can get keeps saying that it’s nitrate, but isn’t nitrate $ N{O_3}^ - $ .
Answer
492.9k+ views
Hint :Yes, this is true that the chemical formula given in the question is the error. No such ion with the chemical formula exists $ N{O^{3 - }} $ in any chemistry books. It is just a mistake. The ion which exists is nitrate ion.
Complete Step By Step Answer:
The ion given in the question $ N{O^{3 - }} $ is a typo. It does not exist in reality. The ion which exists is nitrate ion which has the chemical formula $ N{O_3}^ - $ . Although the chemical formula almost looks the same, we get confused between the two ions. The nitrate ion is a polyatomic ion and the salts which contain this nitrate ion are called nitrates. The nitrate ion is a conjugate base of nitric acid.
The nitrate ion consists of one central atom i.e. nitrogen atom and it is surrounded by three identical bonded oxygen atoms. Its arrangement is trigonal planar. The nitrate ion has a formal charge of $ - 1 $ . These nitrates also show resonance. It has three resonance structures.
Structure of nitrate ion:
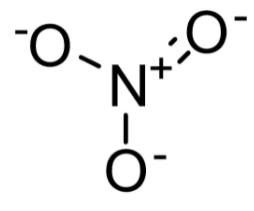
There are many applications of nitrate ions due to their high solubility and biodegradability. It is mainly used as fertilizers in agriculture. Some of the examples of nitrate fertilizer are ammonium, sodium, and magnesium salts etc. It is also used as oxidizing agents in explosives where the oxidation of carbon compounds releases large amounts of gases. Sodium nitrate is used to remove the air bubbles from some ceramics and molten glass.
Hence, no such ion $ N{O^{3 - }} $ exists in chemistry.
Note :
Don’t get confused between these two ions $ N{O^{3 - }} $ and $ N{O_3}^ - $ anions. $ N{O_3}^ - $ is called a nitrate ion whereas $ N{O^{3 - }} $ does not exist. Also, there is a nitride anion $ {N_3}^ - $ , it is the conjugate base of hydrazoic acid $ H{N_3} $ .
Complete Step By Step Answer:
The ion given in the question $ N{O^{3 - }} $ is a typo. It does not exist in reality. The ion which exists is nitrate ion which has the chemical formula $ N{O_3}^ - $ . Although the chemical formula almost looks the same, we get confused between the two ions. The nitrate ion is a polyatomic ion and the salts which contain this nitrate ion are called nitrates. The nitrate ion is a conjugate base of nitric acid.
The nitrate ion consists of one central atom i.e. nitrogen atom and it is surrounded by three identical bonded oxygen atoms. Its arrangement is trigonal planar. The nitrate ion has a formal charge of $ - 1 $ . These nitrates also show resonance. It has three resonance structures.
Structure of nitrate ion:

There are many applications of nitrate ions due to their high solubility and biodegradability. It is mainly used as fertilizers in agriculture. Some of the examples of nitrate fertilizer are ammonium, sodium, and magnesium salts etc. It is also used as oxidizing agents in explosives where the oxidation of carbon compounds releases large amounts of gases. Sodium nitrate is used to remove the air bubbles from some ceramics and molten glass.
Hence, no such ion $ N{O^{3 - }} $ exists in chemistry.
Note :
Don’t get confused between these two ions $ N{O^{3 - }} $ and $ N{O_3}^ - $ anions. $ N{O_3}^ - $ is called a nitrate ion whereas $ N{O^{3 - }} $ does not exist. Also, there is a nitride anion $ {N_3}^ - $ , it is the conjugate base of hydrazoic acid $ H{N_3} $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




