
Name a cyclic unsaturated carbon compound.
Answer
520.6k+ views
Hint: As the name suggests, cyclic compounds have ring structures. Carbon compounds which have some degree of unsaturation are called unsaturated compounds i.e they should contain double or triple bonds.
Complete step by step answer:
Carbon compounds, generally known as organic compounds can be either saturated or unsaturated based on the nature of bonding between the carbon atoms. Those carbon compounds in which all the carbon atoms are connected with single bonds, are known as saturated carbon compounds. On the other hand, carbon compounds in which double or triple bonds between the carbon atoms are present, are called unsaturated carbon compounds. For example, ethane is a saturated carbon compound as both the carbon atoms are singly bonded but ethene is an unsaturated carbon compound because the two carbon atoms are doubly bonded. Here, we can have a look at their structures:
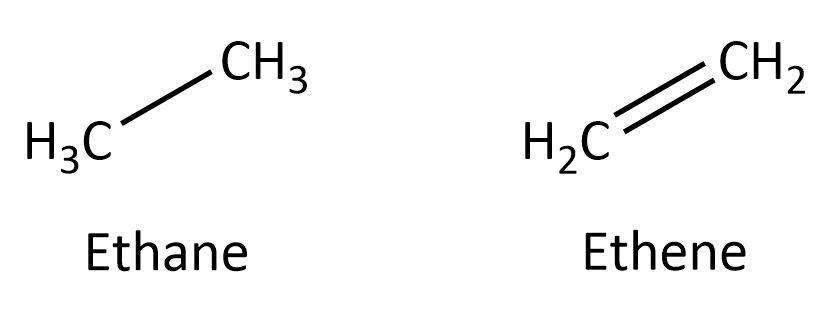
In addition to the above classification, carbon compounds can also be categorized as acyclic and cyclic carbon compounds. In acyclic compounds, carbon atoms are arranged as an open-chain whereas in cyclic compounds, carbon atoms are arranged in a ring structure. For example, in cyclohexane, all the six carbon atoms form a hexagonal ring structure whereas in hexane, the six carbon atoms are arranged as a straight chain. We can look at the structures:
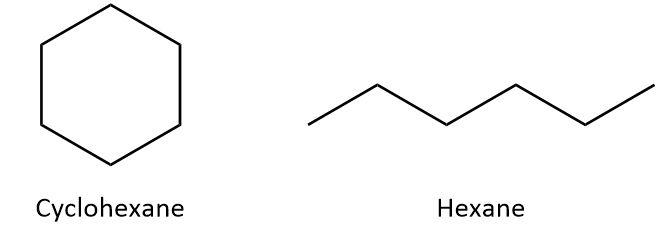
From these two classifications, we can infer that a cyclic unsaturated carbon compound would have ring structure with at least one double or triple bond between the carbon atoms. One such compound is benzene. In benzene, six carbon atoms form a ring structure and there are three single and three double bonds between the carbon atoms. We can draw the structure of benzene as:
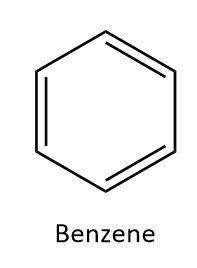
Hence, benzene can be taken as an example of a cyclic unsaturated carbon compound.
Note:
Unsaturated compounds can have single bonds in addition to the double and/or triple bonds but saturated compounds have only single bonds between the carbon atoms.
Complete step by step answer:
Carbon compounds, generally known as organic compounds can be either saturated or unsaturated based on the nature of bonding between the carbon atoms. Those carbon compounds in which all the carbon atoms are connected with single bonds, are known as saturated carbon compounds. On the other hand, carbon compounds in which double or triple bonds between the carbon atoms are present, are called unsaturated carbon compounds. For example, ethane is a saturated carbon compound as both the carbon atoms are singly bonded but ethene is an unsaturated carbon compound because the two carbon atoms are doubly bonded. Here, we can have a look at their structures:
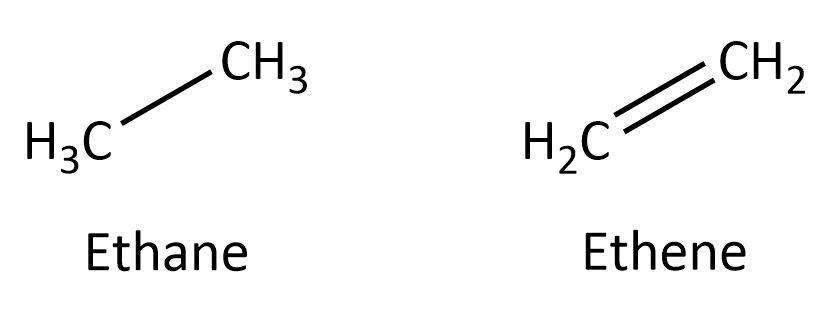
In addition to the above classification, carbon compounds can also be categorized as acyclic and cyclic carbon compounds. In acyclic compounds, carbon atoms are arranged as an open-chain whereas in cyclic compounds, carbon atoms are arranged in a ring structure. For example, in cyclohexane, all the six carbon atoms form a hexagonal ring structure whereas in hexane, the six carbon atoms are arranged as a straight chain. We can look at the structures:

From these two classifications, we can infer that a cyclic unsaturated carbon compound would have ring structure with at least one double or triple bond between the carbon atoms. One such compound is benzene. In benzene, six carbon atoms form a ring structure and there are three single and three double bonds between the carbon atoms. We can draw the structure of benzene as:

Hence, benzene can be taken as an example of a cyclic unsaturated carbon compound.
Note:
Unsaturated compounds can have single bonds in addition to the double and/or triple bonds but saturated compounds have only single bonds between the carbon atoms.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




