
${NAD+}$ and ${NADP+}$ resemble each other in the ability to
a) Give out a proton
b) Take up two electrons at one time
c) Take up two hydrogen atoms
d) Take up one electron at one time
Answer
580.5k+ views
Hint:The functions of ${NAD}{H}$ ${NAD+}$ and ${NADH}$ and ${NADP}{H}$ (${NADP+}$ and ${NADPH}$are undoubtedly significant and distinct. So the regulation of the intracellular balance of ${NAD}{H}$ and ${NADP}{H}$is important The key enzymes involved in the regulation are NAD kinase and NADP phosphatase.
Complete answer:
${NAD}{H}$ is primarily involved in catabolic reactions, while ${NADP}{H}$ participates in anabolic reactions and in defense against oxidative stress. ${NAD}{+}$ functions as a substrate for mono- and poly-ADP ribosylation and is involved in the formation of cyclic ${ADP}$ ribose and in histone deacetylation which is required for transcriptional silencing. Poly ${ADP }$ ribosylation has been implicated in the regulation of several processes, including DNA repair, transcription, and apoptosis.
On the other hand, ${NADP}{+ }$ is a substrate used in the synthesis of nicotinic acid adenine dinucleotide phosphate. This is a potent intracellular ${Ca2}{+}$ mobilizing messenger that triggers ${Ca2}{+ }$ release from lysosomal ${Ca2}{+ }$ stores, which are independent of the stores activated by cyclic ${ ADP}$ ribose and inositol-1,4,5- triphosphate. Considering these significant and distinguishable functions of ${NAD}{H}$ and ${NADP}{H}$, regulation of the intracellular balance of ${NAD}{H}$ and ${NADP}{H}$ is thought to be important in cells.
Additional Information: ${NAD}$ participates in many redox reactions in cells, including those in glycolysis and most of those in the citric acid cycle of cellular respiration.
${NADP}$ is the reducing agent produced by the light reactions of photosynthesis and is consumed in the Calvin cycle of photosynthesis and used in many other anabolic reactions in both plants and animals.
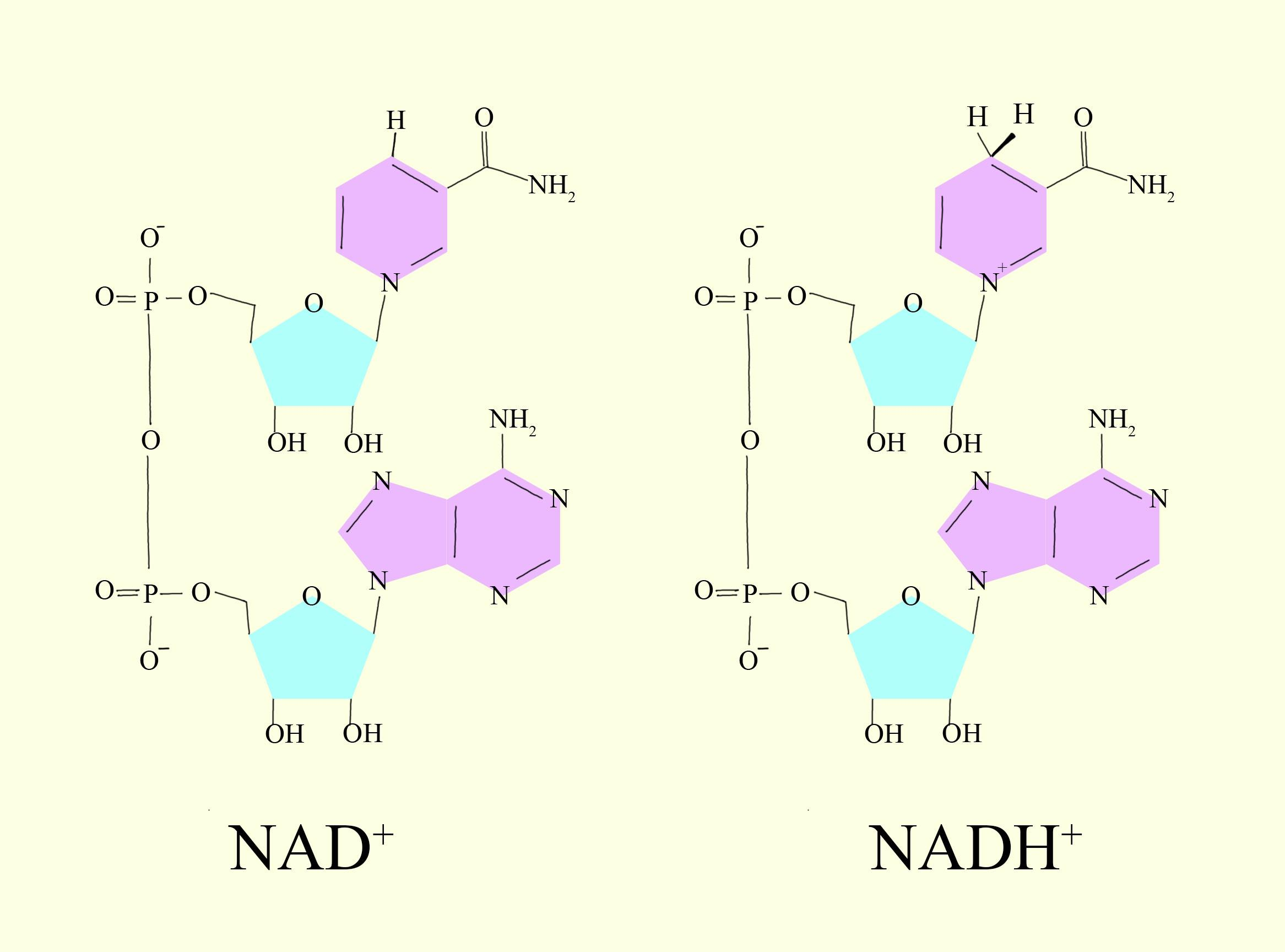
Under the conditions existing in a normal cell, the hydrogen atoms shown in red are dissociated from these acidic substances.
So the correct answer is ‘Take up two electrons at one time ‘.
Note: There have been numerous metabolic engineering studies in which the metabolic network of an organism has been altered by the addition of NAD(P)H-dependent oxidoreductase(s), or pathways including them. One of the most frequent goals of this type of approach is the efficient production of a given chemical of interest without the need for chemical synthesis or purifying enzymes, and ideally from renewable feedstocks and with a lower energy consumption due to the mild conditions at which reactions can be catalyzed by ${ NAD}{P}{H}$ dependent oxidoreductases.
Complete answer:
${NAD}{H}$ is primarily involved in catabolic reactions, while ${NADP}{H}$ participates in anabolic reactions and in defense against oxidative stress. ${NAD}{+}$ functions as a substrate for mono- and poly-ADP ribosylation and is involved in the formation of cyclic ${ADP}$ ribose and in histone deacetylation which is required for transcriptional silencing. Poly ${ADP }$ ribosylation has been implicated in the regulation of several processes, including DNA repair, transcription, and apoptosis.
On the other hand, ${NADP}{+ }$ is a substrate used in the synthesis of nicotinic acid adenine dinucleotide phosphate. This is a potent intracellular ${Ca2}{+}$ mobilizing messenger that triggers ${Ca2}{+ }$ release from lysosomal ${Ca2}{+ }$ stores, which are independent of the stores activated by cyclic ${ ADP}$ ribose and inositol-1,4,5- triphosphate. Considering these significant and distinguishable functions of ${NAD}{H}$ and ${NADP}{H}$, regulation of the intracellular balance of ${NAD}{H}$ and ${NADP}{H}$ is thought to be important in cells.
Additional Information: ${NAD}$ participates in many redox reactions in cells, including those in glycolysis and most of those in the citric acid cycle of cellular respiration.
${NADP}$ is the reducing agent produced by the light reactions of photosynthesis and is consumed in the Calvin cycle of photosynthesis and used in many other anabolic reactions in both plants and animals.
Under the conditions existing in a normal cell, the hydrogen atoms shown in red are dissociated from these acidic substances.
So the correct answer is ‘Take up two electrons at one time ‘.

Note: There have been numerous metabolic engineering studies in which the metabolic network of an organism has been altered by the addition of NAD(P)H-dependent oxidoreductase(s), or pathways including them. One of the most frequent goals of this type of approach is the efficient production of a given chemical of interest without the need for chemical synthesis or purifying enzymes, and ideally from renewable feedstocks and with a lower energy consumption due to the mild conditions at which reactions can be catalyzed by ${ NAD}{P}{H}$ dependent oxidoreductases.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




