
$n - $ Butane and $iso - $ Butane are ?
Choose the correct option:
(A) Same compounds
(B) Allotropes
(C) Isomers
(D) Structural isomers
Answer
572.4k+ views
Hint:We have studied about catenation. Maximum catenation is shown by carbon in the periodic table because of its small size. Butane is an alkane in which four carbon atoms are bonded with single covalent bonds, both $n - $ Butane and $iso - $ Butane have a molecular formula ${C_4}{H_{10}}$ but the arrangement is different.
Complete answer:We have studied about catenation. Catenation is a process in which the same element forms bonds with itself and forms a chain or a ring molecule. The bond in catenation is covalent. A covalent bond is formed when electrons are shared between atoms. Catenation is shown by elements like sulfur and carbon. Maximum catenation is shown by carbon in the periodic table because of its small size.
Butane is an alkane in which four carbon atoms are bonded with single covalent bonds. Alkane has a molecular formula ${C_n}{H_{2n + 2}}$ . When a hydrocarbon has carbon atoms bonded with a double covalent bond, it is called alkene, molecular formula ${C_n}{H_{2n}}$ . Similarly, if a hydrocarbon has carbon atoms bonded with a triple covalent bond, it is called alkyne, molecular formula ${C_n}{H_{2n - 2}}$ .
Here we are given $n - $ butane and $iso - $ butane. Both have a molecular formula ${C_4}{H_{10}}$ . $n - $ Butane is written as:
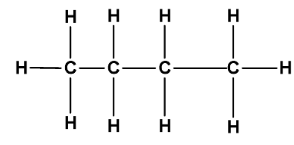
Here $n - $ is a prefix that tells us that the chain is continuous and unbranched. $iso - $ Butane is written as:
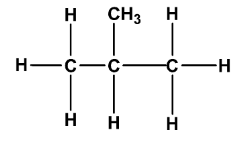
Here $iso - $ prefix means that all carbon atoms except one are continuous. Notice that the number of carbon and hydrogen atoms are the same in both structures, only the arrangement is different. Such structures are called structural isomers.
Hence, the correct option is $(D)$.
Note:It is important to know that the $IUPAC$ name of $iso - $ butane is $2 - $ methylpropane. We know that there are certain steps in naming a hydrocarbon. First, we identify the longest continuous chain and number it consecutively. The number is assigned in such a way that the branched carbon atom gets the lowest possible number.
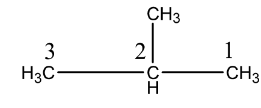
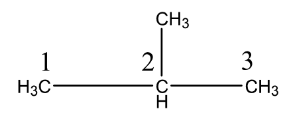
Or we can also write
Complete answer:We have studied about catenation. Catenation is a process in which the same element forms bonds with itself and forms a chain or a ring molecule. The bond in catenation is covalent. A covalent bond is formed when electrons are shared between atoms. Catenation is shown by elements like sulfur and carbon. Maximum catenation is shown by carbon in the periodic table because of its small size.
Butane is an alkane in which four carbon atoms are bonded with single covalent bonds. Alkane has a molecular formula ${C_n}{H_{2n + 2}}$ . When a hydrocarbon has carbon atoms bonded with a double covalent bond, it is called alkene, molecular formula ${C_n}{H_{2n}}$ . Similarly, if a hydrocarbon has carbon atoms bonded with a triple covalent bond, it is called alkyne, molecular formula ${C_n}{H_{2n - 2}}$ .
Here we are given $n - $ butane and $iso - $ butane. Both have a molecular formula ${C_4}{H_{10}}$ . $n - $ Butane is written as:

Here $n - $ is a prefix that tells us that the chain is continuous and unbranched. $iso - $ Butane is written as:

Here $iso - $ prefix means that all carbon atoms except one are continuous. Notice that the number of carbon and hydrogen atoms are the same in both structures, only the arrangement is different. Such structures are called structural isomers.
Hence, the correct option is $(D)$.
Note:It is important to know that the $IUPAC$ name of $iso - $ butane is $2 - $ methylpropane. We know that there are certain steps in naming a hydrocarbon. First, we identify the longest continuous chain and number it consecutively. The number is assigned in such a way that the branched carbon atom gets the lowest possible number.

Or we can also write

Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




