
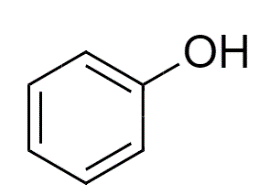
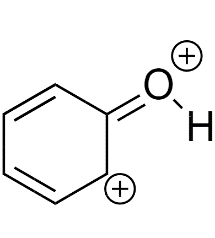
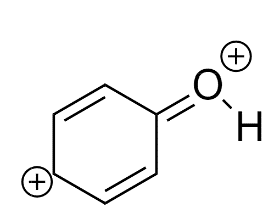
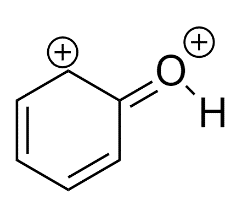
Most unstable resonating structure of phenol is :
A)
B)
C)
D)




Answer
539.7k+ views
Hint: Before solving this question, we should know about the resonance, resonating structures of phenol and then find out which one of them is less stable. Resonating structures are the lewis structures of the same compound. They are hybrid structures whose positions of electrons are different but the position of the atom is the same.
Complete answer:
The derivative of aromatic hydrocarbon which has a hydroxyl group, are known as phenols. The molecular formula of it is ${{C}_{6}}{{H}_{5}}OH$. The other names of phenol are benzenol, carbolic acid. It is a volatile solid compound that is white or colorless.
Phenol is represented by four Resonating structures. Resonance helps to stabilize the phenol as well as the phenoxide ion by delocalizing the electrons in the ring. The oxygen atom becomes positive because of the separation of charge that occurs due to the delocalization in phenol whereas in phenoxide ion the delocalization makes it more stable because charge separation does not occur in this. If we have to compare which is the less stable resonating structure, that would be Phenol.
So, Option (A) Phenol
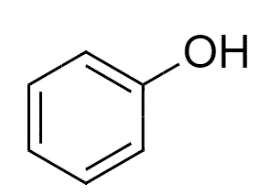 is the correct option.
is the correct option.
So, the correct answer is “Option A”.
Note:
The process of delocalizing the electrons in molecules where the bonding cannot be described by just one lewis structure is known as Resonance. In Resonance, Every lewis structure contributes to the formation of the target molecule or ion. One should not mistake the lewis structures being the isomers as only the position of delocalized electrons differs. It is also known as mesomerism.
Complete answer:
The derivative of aromatic hydrocarbon which has a hydroxyl group, are known as phenols. The molecular formula of it is ${{C}_{6}}{{H}_{5}}OH$. The other names of phenol are benzenol, carbolic acid. It is a volatile solid compound that is white or colorless.
Phenol is represented by four Resonating structures. Resonance helps to stabilize the phenol as well as the phenoxide ion by delocalizing the electrons in the ring. The oxygen atom becomes positive because of the separation of charge that occurs due to the delocalization in phenol whereas in phenoxide ion the delocalization makes it more stable because charge separation does not occur in this. If we have to compare which is the less stable resonating structure, that would be Phenol.
So, Option (A) Phenol

So, the correct answer is “Option A”.
Note:
The process of delocalizing the electrons in molecules where the bonding cannot be described by just one lewis structure is known as Resonance. In Resonance, Every lewis structure contributes to the formation of the target molecule or ion. One should not mistake the lewis structures being the isomers as only the position of delocalized electrons differs. It is also known as mesomerism.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




