
What is the molecular shape geometry of $CHClO$?
Answer
535.2k+ views
Hint: Molecular geometry is generally a three-dimensional arrangement of the atoms that makes a molecule. Molecular geometry includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters which tells us about the position of each atom.
Complete answer:
VSEPR theory is basically used to predict the geometry of molecules this theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom.
VSEPR is the abbreviation of Valence Shell Electron Pair Repulsion theory. This theory is basically based on the concept that there is repulsion between the pairs of valence electrons where valence electrons are those electrons which are present in the outermost shell of electron and this theory was presented by Sidgwick and Powell in 1940.
The given compound is known by the name formyl chloride and it can be shown as:
$O=C(Cl)-H$and suggests that it is trigonal planar this explained on the basis of 3 regions of electron density around the carbon which has a formal$p\pi -p\pi $ $CO$ bond and the two bond angles $\angle Cl-C-O$and $\angle H-C-O$are $120{}^\circ $which shows that it has trigonal planar geometry which can be shown as:
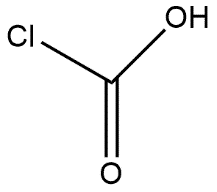
Hence the molecular shape geometry of $CHClO$ is trigonal planar.
Note:
Mainly VSEPR theory tells us about the bonding and molecular geometry of organic molecules and polyatomic ions. It predicts the 3-D shape of the molecule and it can predict the shape of nearly all compounds.
Complete answer:
VSEPR theory is basically used to predict the geometry of molecules this theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom.
VSEPR is the abbreviation of Valence Shell Electron Pair Repulsion theory. This theory is basically based on the concept that there is repulsion between the pairs of valence electrons where valence electrons are those electrons which are present in the outermost shell of electron and this theory was presented by Sidgwick and Powell in 1940.
The given compound is known by the name formyl chloride and it can be shown as:
$O=C(Cl)-H$and suggests that it is trigonal planar this explained on the basis of 3 regions of electron density around the carbon which has a formal$p\pi -p\pi $ $CO$ bond and the two bond angles $\angle Cl-C-O$and $\angle H-C-O$are $120{}^\circ $which shows that it has trigonal planar geometry which can be shown as:

Hence the molecular shape geometry of $CHClO$ is trigonal planar.
Note:
Mainly VSEPR theory tells us about the bonding and molecular geometry of organic molecules and polyatomic ions. It predicts the 3-D shape of the molecule and it can predict the shape of nearly all compounds.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




