
What is the molecular geometry of $ S{{I}_{6}} $ ? Draw its VSEPR and Lewis structure.
Answer
521.4k+ views
Hint :We know that for solving the given problem, the knowledge of VSEPR and Lewis structure should be present. Lewis structure helps in representing valence electrons of a molecule. On calculating, the formal charges with respect to the Lewis structure, the charge on a polyatomic ion can be defined easily. VSEPR theory is the valence shell electron pair repulsion theory. It helps in the prediction of the geometrical structure of the compound.
Complete Step By Step Answer:
Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure. If the remainder is $ 1 $ , then $ l.p=1 $ and if the remainder is $ 2 $ and greater than $ 2 $ , then it should be divided by $ 2 $ to get the quotient as a lone pair.
Step-1: The molecular geometry of the compound. is identified using VSEPR theory. The rules for VSEPR are given :
Simple Lewis structure is obtained.
Bonds between $ 2 $ atoms are always considered $ 1 $ .
Here, geometry is total ; Electron pair = bond pair + lone pair
Step-2: By adding the total number of valence electrons, we get $ 6 $ valence electron of Sulphur and $ 7 $ valence electron of Iodine. So, total valence electrons $ =6+7\times 6=48 $ valence electron.
Step-3; By dividing total valence electrons by $ 8 $ , we get the bond pair as quotient.
So, $ 48\div 8=6 $ bond pair.
Since, there is no remainder, so, $ l.p=0 $ .
Step-4: When $ e.p=l.p+b.p=6 $ , the shape obtained is octahedral.
The Lewis structure of $ S{{I}_{6}} $ is given as follows :
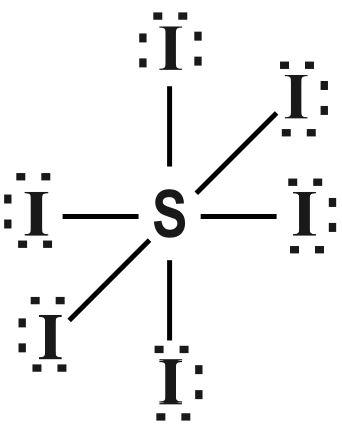
Note :
Remember that VSEPR along with geometrical structure also determines the shape of the molecule. The electrons in VSEPR occupy the positions that make the repulsion less. : VSEPR theory is basically used to predict the shape of molecules by using systematic steps. Whereas, lewis structure is also known as lewis dots, it is a representation of valence electrons in a given molecule.
Complete Step By Step Answer:
Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure. If the remainder is $ 1 $ , then $ l.p=1 $ and if the remainder is $ 2 $ and greater than $ 2 $ , then it should be divided by $ 2 $ to get the quotient as a lone pair.
Step-1: The molecular geometry of the compound. is identified using VSEPR theory. The rules for VSEPR are given :
Simple Lewis structure is obtained.
Bonds between $ 2 $ atoms are always considered $ 1 $ .
Here, geometry is total ; Electron pair = bond pair + lone pair
Step-2: By adding the total number of valence electrons, we get $ 6 $ valence electron of Sulphur and $ 7 $ valence electron of Iodine. So, total valence electrons $ =6+7\times 6=48 $ valence electron.
Step-3; By dividing total valence electrons by $ 8 $ , we get the bond pair as quotient.
So, $ 48\div 8=6 $ bond pair.
Since, there is no remainder, so, $ l.p=0 $ .
Step-4: When $ e.p=l.p+b.p=6 $ , the shape obtained is octahedral.
The Lewis structure of $ S{{I}_{6}} $ is given as follows :

Note :
Remember that VSEPR along with geometrical structure also determines the shape of the molecule. The electrons in VSEPR occupy the positions that make the repulsion less. : VSEPR theory is basically used to predict the shape of molecules by using systematic steps. Whereas, lewis structure is also known as lewis dots, it is a representation of valence electrons in a given molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




