
What is the molecular geometry of $ {H_2}O $ ? Draw its VSEPR structure.
Answer
528k+ views
Hint: VSEPR theory basically shows the molecular shapes, the full form is valence shell electron pair repulsion. The basic steps are find the central atom then count its valence electrons after that add one electron for each bonding atom then add or subtract electrons for charge at the end divide it by two to find total number of electron pairs and this number will help us to predict the geometry of the shape.
Complete answer:
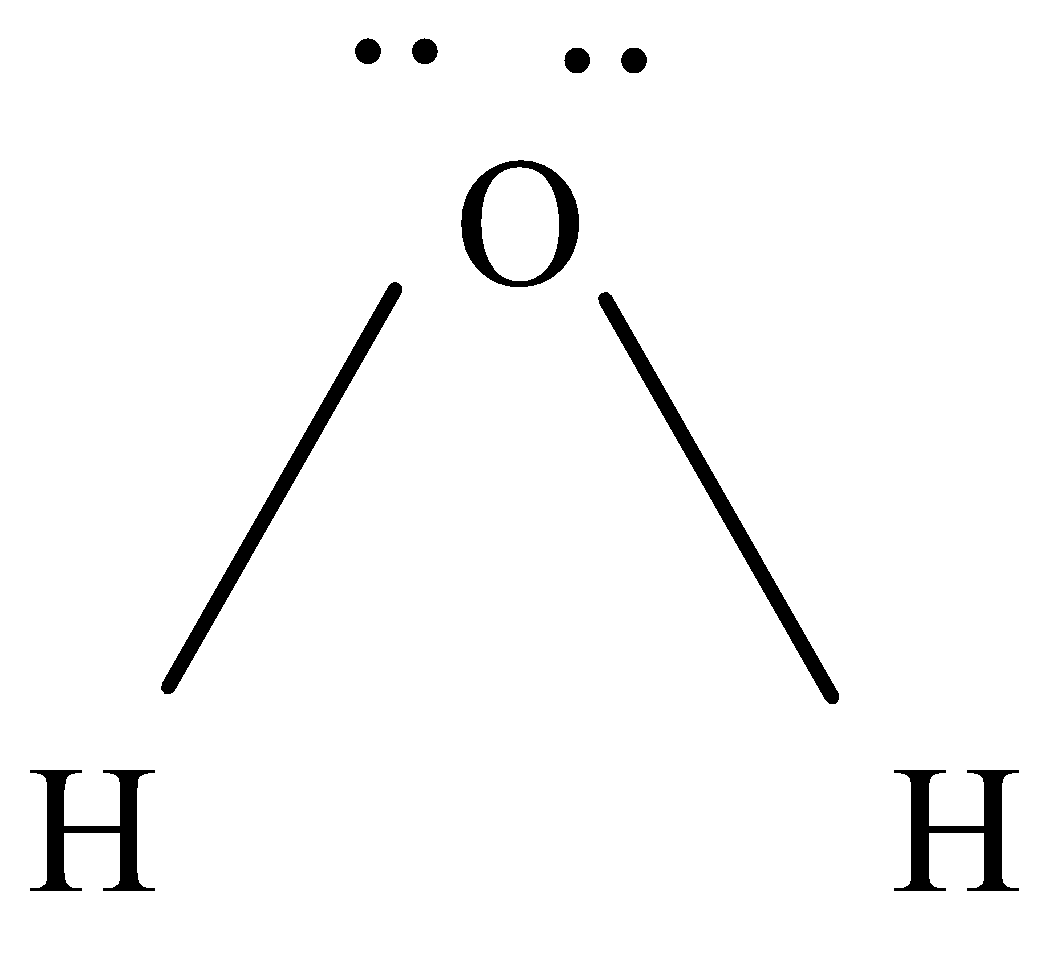
To know the molecular geometry we will draw lewis structure.
As we know we have two hydrogen ions and one oxygen, oxygen will be at the centre.
So, the central bond has two bonds with hydrogen and two lone pairs.
They are arranged in tetrahedral shape. The angle of $ H - O - H $ is $ {104.5^o} $
So basically, water has bent geometry or V-shaped geometry due to the presence of lone pairs. The hybridization of water is $ s{p^3} $ .
Note:
The V-shape either occurs due to lone pair-lone pair, lone pair-bond pair or bond pair- bond pair repulsion. Understanding the molecular structure of any compound helps us to understand polarity, reactivity, magnetism and many other properties.
Complete answer:
To know the molecular geometry we will draw lewis structure.
As we know we have two hydrogen ions and one oxygen, oxygen will be at the centre.
So, the central bond has two bonds with hydrogen and two lone pairs.
They are arranged in tetrahedral shape. The angle of $ H - O - H $ is $ {104.5^o} $
So basically, water has bent geometry or V-shaped geometry due to the presence of lone pairs. The hybridization of water is $ s{p^3} $ .

Note:
The V-shape either occurs due to lone pair-lone pair, lone pair-bond pair or bond pair- bond pair repulsion. Understanding the molecular structure of any compound helps us to understand polarity, reactivity, magnetism and many other properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




