
What is the molecular geometry of $ BrF_{4}^{-} $ ?
(A) square planar
(B) square pyramidal
(C) seesaw
(D) tetrahedral
Answer
537.6k+ views
Hint :Prediction of structures of compounds can be done through the VSEPR (Valence-shell electron-pair repulsion) model. This model is based on the idea that the lowest-energy arrangement for a compound is the one in which in its electron pairs (bonding and non-bonding) are as far as possible.
Complete Step By Step Answer:
The VSEPR model can help us predict the structure of nearly any molecule or polyatomic ion in which the central atom is a non-metal, as well as the structures of many molecules and polyatomic ions with a central metal ion. Bromine trifluoride $ \left( BrF_{4}^{-} \right) $ is an interhalogen compound of straw-colored liquid with a pungent odor. $ \left( BrF_{4}^{-} \right) $ Contains four bonded and two bonded electron domains giving a T-shaped and planar molecular geometry.
Start from the Lewis structure of the tetrafluoroborate ion, $ BrF_{4}^{-} $ . The molecule will have a total of $ 36 $ valence electrons $ -~7~ $ from bromine, $ 7 $ from each of the four fluorine atoms, and one extra electron to give the ion the $ -1 $ charge.
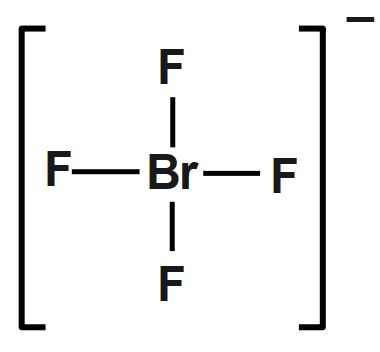
The bromine atom will be bonded to each of the four fluorine atoms via single bonds for a total of eight of the 36 valence electrons. The fluorine atoms will each have three lone pairs attached, bringing the number of used valence electrons to $ ~32. $ The remaining four valence electrons will be placed on bromine as lone pairs. The bromine atom is surrounded by six regions of electron density - four single bonds and two lone pairs, which means that its steric number will be equal to six.
Therefore, the correct answer is option A.
Note :
Be careful while counting the number of electrons for calculations. One important thing to note is that coordination number four can show both tetrahedral as well as square planar geometry. If the central metal atom is showing hybridization, it will exhibit square planar geometry. If the central metal atom is hybridized, it will exhibit tetrahedral geometry
Complete Step By Step Answer:
The VSEPR model can help us predict the structure of nearly any molecule or polyatomic ion in which the central atom is a non-metal, as well as the structures of many molecules and polyatomic ions with a central metal ion. Bromine trifluoride $ \left( BrF_{4}^{-} \right) $ is an interhalogen compound of straw-colored liquid with a pungent odor. $ \left( BrF_{4}^{-} \right) $ Contains four bonded and two bonded electron domains giving a T-shaped and planar molecular geometry.
Start from the Lewis structure of the tetrafluoroborate ion, $ BrF_{4}^{-} $ . The molecule will have a total of $ 36 $ valence electrons $ -~7~ $ from bromine, $ 7 $ from each of the four fluorine atoms, and one extra electron to give the ion the $ -1 $ charge.

The bromine atom will be bonded to each of the four fluorine atoms via single bonds for a total of eight of the 36 valence electrons. The fluorine atoms will each have three lone pairs attached, bringing the number of used valence electrons to $ ~32. $ The remaining four valence electrons will be placed on bromine as lone pairs. The bromine atom is surrounded by six regions of electron density - four single bonds and two lone pairs, which means that its steric number will be equal to six.
Therefore, the correct answer is option A.
Note :
Be careful while counting the number of electrons for calculations. One important thing to note is that coordination number four can show both tetrahedral as well as square planar geometry. If the central metal atom is showing hybridization, it will exhibit square planar geometry. If the central metal atom is hybridized, it will exhibit tetrahedral geometry
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Explain zero factorial class 11 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

State and prove Bernoullis theorem class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




