
What is the molecular formula of mCPBA (meta-chloroperoxybenzoic acid)?
(A)- ${{C}_{7}}{{O}_{4}}{{H}_{4}}Cl$
(B)- ${{C}_{6}}{{O}_{2}}{{H}_{5}}Cl$
(C)- ${{C}_{7}}{{O}_{2}}{{H}_{5}}Cl$
(D)- ${{C}_{7}}{{O}_{3}}{{H}_{5}}Cl$
Answer
591.3k+ views
Hint: Molecular formula of a compound indicates the number of atoms of each element present in a molecule of the compound.
meta-chloroperoxybenzoic acid commonly referred to as mCPBA is a peroxycarboxylic acid. It can be considered as a derivative of meta-chlorobenzoic acid.
Complete answer:
Let us try to deduce the molecular formula of mCPBA (meta-chloroperoxybenzoic acid).
As the name suggests it is a peroxy acid. Peroxy acids have one $O-O$ bond and can generally be represented as
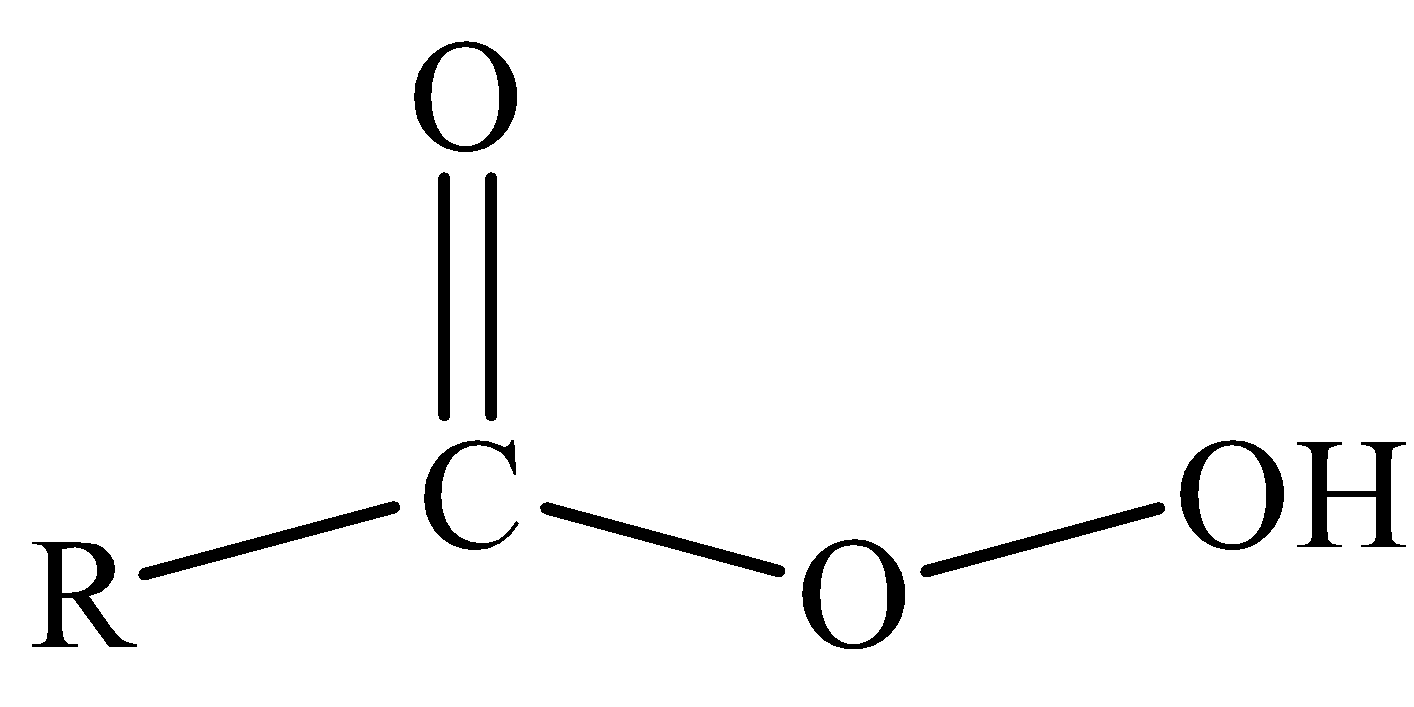
If we replace R-group in the above structure of peroxy acid by meta-substituted chlorobenzene, then the structure we obtain is of meta-chloroperoxybenzoic acid.
Structure of mCPBA (meta-chloroperoxybenzoic acid) is shown below:
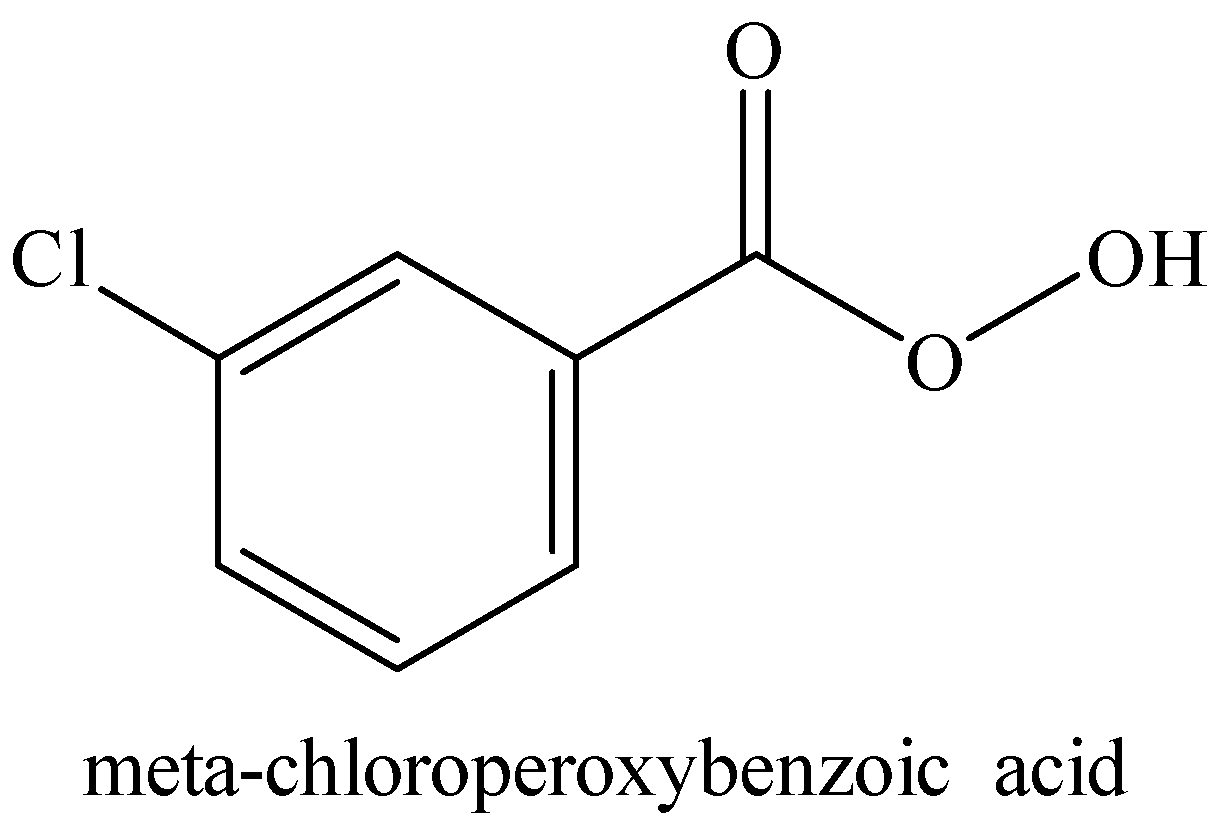
Let us calculate the number of each type of atoms from the structure of mCPBA.
Number of carbon atoms, C = 6 carbon atoms in benzene ring + 1 carbonyl carbon = 7
Number of oxygen atoms = 1 oxygen of carbonyl group + 2 oxygen of peroxide bond = 3
Number of hydrogen atoms = 4 hydrogen on benzene ring + 1 hydrogen bonded with oxygen = 5
There is only one chlorine atom.
Therefore, we can use the molecular formula of mCPBA as ${{C}_{7}}{{O}_{3}}{{H}_{5}}Cl$.
Hence, the correct option will be (D).
Additional information:
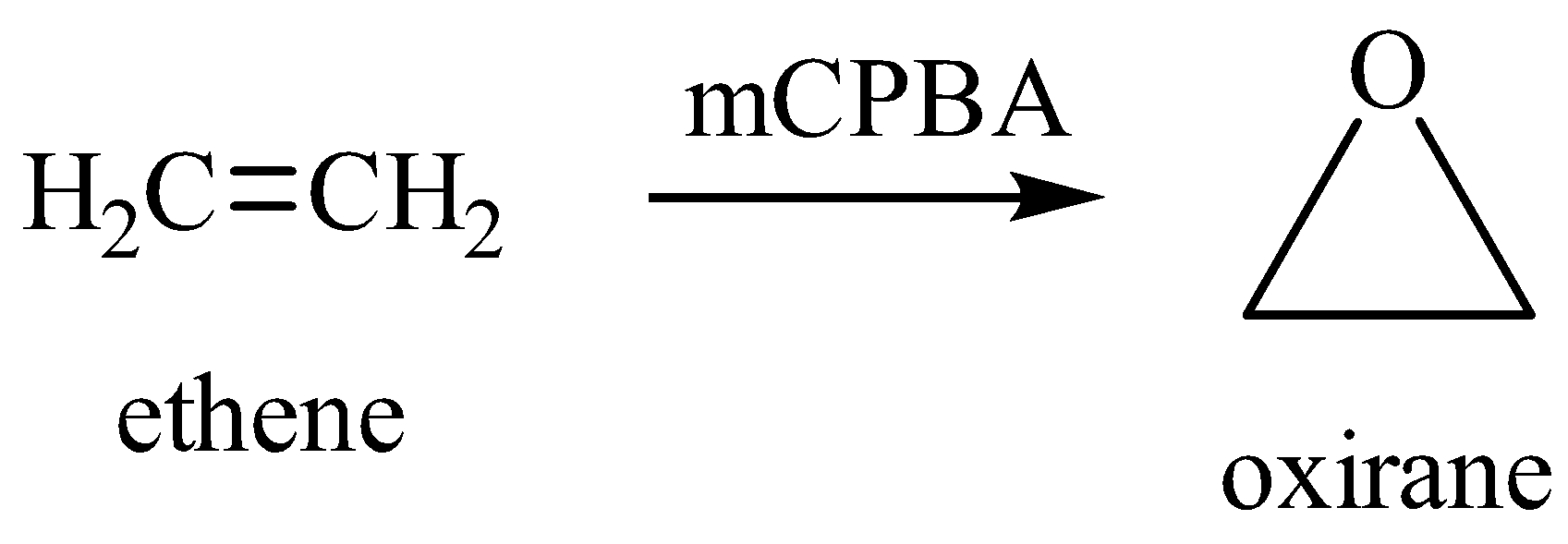
mCPBA is a very good oxidizing agent and preferably used in epoxide formation reactions. Epoxides are cylcoethers. Alkenes are converted to epoxides by mCPBA and meta-chlorobenzoic acid is formed as a by-product in the reaction.
It is prepared by the reaction of meta-chlorobenzoyl chloride with an alkaline solution of hydrogen peroxide (${{H}_{2}}{{O}_{2}}$).
Note:
Carefully count the number of atoms of each kind. It is better to write the structure before calculating the number of atoms. Keep in mind that the valency of carbon is four and there is one hydrogen at each carbon on the ring except for positions which are substituted.
meta-chloroperoxybenzoic acid commonly referred to as mCPBA is a peroxycarboxylic acid. It can be considered as a derivative of meta-chlorobenzoic acid.
Complete answer:
Let us try to deduce the molecular formula of mCPBA (meta-chloroperoxybenzoic acid).
As the name suggests it is a peroxy acid. Peroxy acids have one $O-O$ bond and can generally be represented as

If we replace R-group in the above structure of peroxy acid by meta-substituted chlorobenzene, then the structure we obtain is of meta-chloroperoxybenzoic acid.
Structure of mCPBA (meta-chloroperoxybenzoic acid) is shown below:

Let us calculate the number of each type of atoms from the structure of mCPBA.
Number of carbon atoms, C = 6 carbon atoms in benzene ring + 1 carbonyl carbon = 7
Number of oxygen atoms = 1 oxygen of carbonyl group + 2 oxygen of peroxide bond = 3
Number of hydrogen atoms = 4 hydrogen on benzene ring + 1 hydrogen bonded with oxygen = 5
There is only one chlorine atom.
Therefore, we can use the molecular formula of mCPBA as ${{C}_{7}}{{O}_{3}}{{H}_{5}}Cl$.
Hence, the correct option will be (D).
Additional information:
mCPBA is a very good oxidizing agent and preferably used in epoxide formation reactions. Epoxides are cylcoethers. Alkenes are converted to epoxides by mCPBA and meta-chlorobenzoic acid is formed as a by-product in the reaction.

It is prepared by the reaction of meta-chlorobenzoyl chloride with an alkaline solution of hydrogen peroxide (${{H}_{2}}{{O}_{2}}$).
Note:
Carefully count the number of atoms of each kind. It is better to write the structure before calculating the number of atoms. Keep in mind that the valency of carbon is four and there is one hydrogen at each carbon on the ring except for positions which are substituted.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




