
Methyl magnesium bromide is treated with carbon dioxide and hydrolysed; the product is:
A.Ethyl alcohol
B.Diethyl carbonate
C.Acetic acid
D.Propanoic acid
Answer
576.3k+ views
Hint: Carbonation of Grignard reagent is the reaction of Grignard reagent with \[C{O_2}\]. So, when \[C{O_2}\] reacts with organometallic reagents (Grignard reagents), we are familiar with the attack of R- anion on carbonyl groups, i.e., an acid salt is formed. This acid salt is further hydrolyzed in the presence of acid to form a carboxylic acid. These acids can then be used for various purposes.

Complete step by step answer:
Grignard reagents react with carbon dioxide in two stages, in the first stage, we will get an addition of the Grignard reagent to the carbon dioxide. Dry carbon dioxide is bubbled through a solution of the Grignard reagent in ethoxyethane.
So, when Methyl magnesium bromide is treated with carbon dioxide, we know that \[C{O_2}\] has two \[C = O\] bonds. The anion attacks the carbon and forms an acid salt. Carbon dioxide gets added to the Methyl magnesium bromide in the reaction shown below.
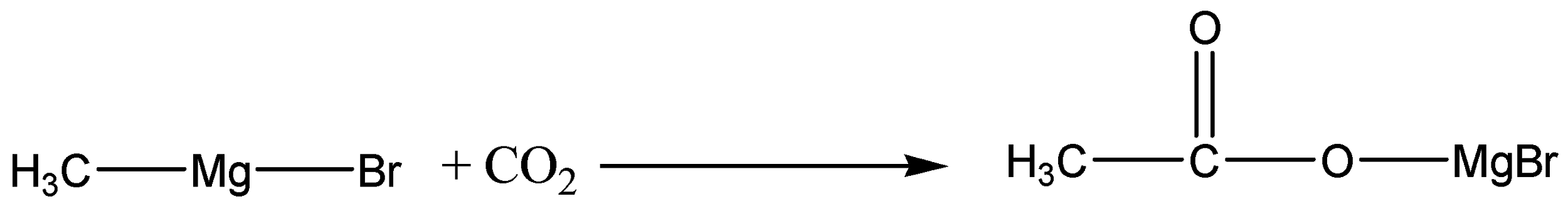
The product is then hydrolysed or it is reacted with water in the presence of a dilute acid. Typically, we add dilute sulphuric acid or dilute hydrochloric acid to the solution formed by the reaction with the \[C{O_2}\]. A carboxylic acid is produced with one more carbon than the original Grignard reagent.
In this case, acetic acid is formed as the product.

So, when Methyl magnesium bromide is treated with carbon dioxide and hydrolysed, the product is Acetic acid.
Therefore, the correct answer is option (C).
Note: A Grignard reagent of Grignard compound is a chemical compound with the generic formula R−Mg−X, where X is a halogen and R is an organic group, it can be an alkyl or aryl normally.

Complete step by step answer:
Grignard reagents react with carbon dioxide in two stages, in the first stage, we will get an addition of the Grignard reagent to the carbon dioxide. Dry carbon dioxide is bubbled through a solution of the Grignard reagent in ethoxyethane.
So, when Methyl magnesium bromide is treated with carbon dioxide, we know that \[C{O_2}\] has two \[C = O\] bonds. The anion attacks the carbon and forms an acid salt. Carbon dioxide gets added to the Methyl magnesium bromide in the reaction shown below.

The product is then hydrolysed or it is reacted with water in the presence of a dilute acid. Typically, we add dilute sulphuric acid or dilute hydrochloric acid to the solution formed by the reaction with the \[C{O_2}\]. A carboxylic acid is produced with one more carbon than the original Grignard reagent.
In this case, acetic acid is formed as the product.

So, when Methyl magnesium bromide is treated with carbon dioxide and hydrolysed, the product is Acetic acid.
Therefore, the correct answer is option (C).
Note: A Grignard reagent of Grignard compound is a chemical compound with the generic formula R−Mg−X, where X is a halogen and R is an organic group, it can be an alkyl or aryl normally.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




