
What is meant by fractional distillation? How does it differ from simple distillation? What part of the fractional distillation apparatus makes it more efficient and possesses an advantage over simple distillation processes? Explain using a diagram.
Give one example of fractional distillation in industry.
Answer
514.8k+ views
Hint: there are various techniques used for the separation of organic compounds. Distillation is a technique used to separate a mixture of organic compounds. Fractional distillation is a more efficient technique to separate various organic mixtures. It uses a fractional column that differs from simple distillation.
Complete answer:
There are methods applied to separate the mixtures of various organic solutions that are volatile or non – volatile. A method that is used to separate mixtures of volatile solutions having less difference in boiling points is fractional distillation. This consists of a fractionating column. The vapors of the liquid with less boiling point are vaporized first and then by successive vaporization and condensation the mixtures get separated.
Fractional distillation is different from simple distillation as simple distillation is suitable for separating volatile liquids from non – volatile solvents as they have sufficient difference in their boiling points, also the apparatus does not use a fractionating column.
Fractional distillation is more efficient from simple distillation as it possess a fractionating column on the mouth of the flask. This column has beads and is capable for successive condensation and vaporization called as theoretical plates in the fractionating column. This provides a more surface area for cooling and efficiency in separating solutions according to their boiling point differences. The diagram of a fractional distillation apparatus is:
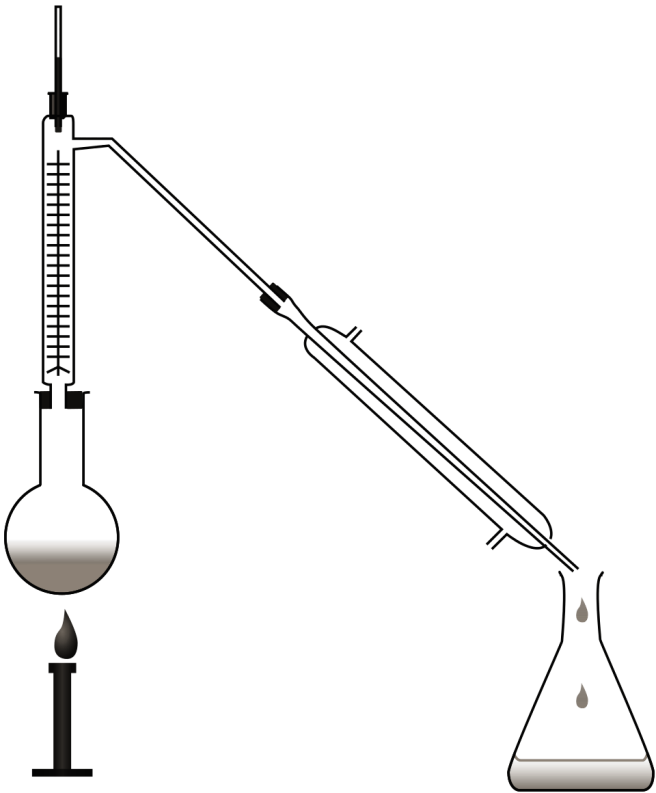
An example of fractional distillation used in the industry is the distillation of crude oil in the petroleum industry.
Note:
There are some other distillation techniques apart from simple and fractional distillation like distillation under reduced pressure that is used to purify liquids having very high boiling points or for liquids that decompose below their boiling point, and steam distillation used to purify liquids that are steam volatile.
Complete answer:
There are methods applied to separate the mixtures of various organic solutions that are volatile or non – volatile. A method that is used to separate mixtures of volatile solutions having less difference in boiling points is fractional distillation. This consists of a fractionating column. The vapors of the liquid with less boiling point are vaporized first and then by successive vaporization and condensation the mixtures get separated.
Fractional distillation is different from simple distillation as simple distillation is suitable for separating volatile liquids from non – volatile solvents as they have sufficient difference in their boiling points, also the apparatus does not use a fractionating column.
Fractional distillation is more efficient from simple distillation as it possess a fractionating column on the mouth of the flask. This column has beads and is capable for successive condensation and vaporization called as theoretical plates in the fractionating column. This provides a more surface area for cooling and efficiency in separating solutions according to their boiling point differences. The diagram of a fractional distillation apparatus is:

An example of fractional distillation used in the industry is the distillation of crude oil in the petroleum industry.
Note:
There are some other distillation techniques apart from simple and fractional distillation like distillation under reduced pressure that is used to purify liquids having very high boiling points or for liquids that decompose below their boiling point, and steam distillation used to purify liquids that are steam volatile.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




