
What is meant by an acetal? Give an example of a reaction(with mechanism) showing its formation.
Answer
504.3k+ views
Hint :The word ketal is occasionally used to refer to structures that are linked to ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes, whereas the term acetal was formerly used to refer to structures that were related to aldehydes (having at least one hydrogen in place of an R on the central carbon). The International Union of Pure and Applied Chemistry (IUPAC) first discouraged the use of the term ketal, but has subsequently altered its position. Ketals are now a subset of acetals, a word that now covers both aldehyde- and ketone-derived compounds, in contrast to past use.
Complete Step By Step Answer:
A functional group with the connectivity $ {R_2}C{\left( {OR'} \right)_2} $ is known as an acetal. The R groups in this case can be organic fragments (a carbon atom with various additional atoms attached) or hydrogen, but the R' groups must be organic fragments and not hydrogen. The two R' groups may or may not be equal (a "symmetric acetal") (a "mixed acetal"). Acetals are produced from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon as carbonyl compounds, but have significantly different chemical stability and reactivity. Because the centre carbon atom is saturated and possesses tetrahedral geometry, it contains four bonds.
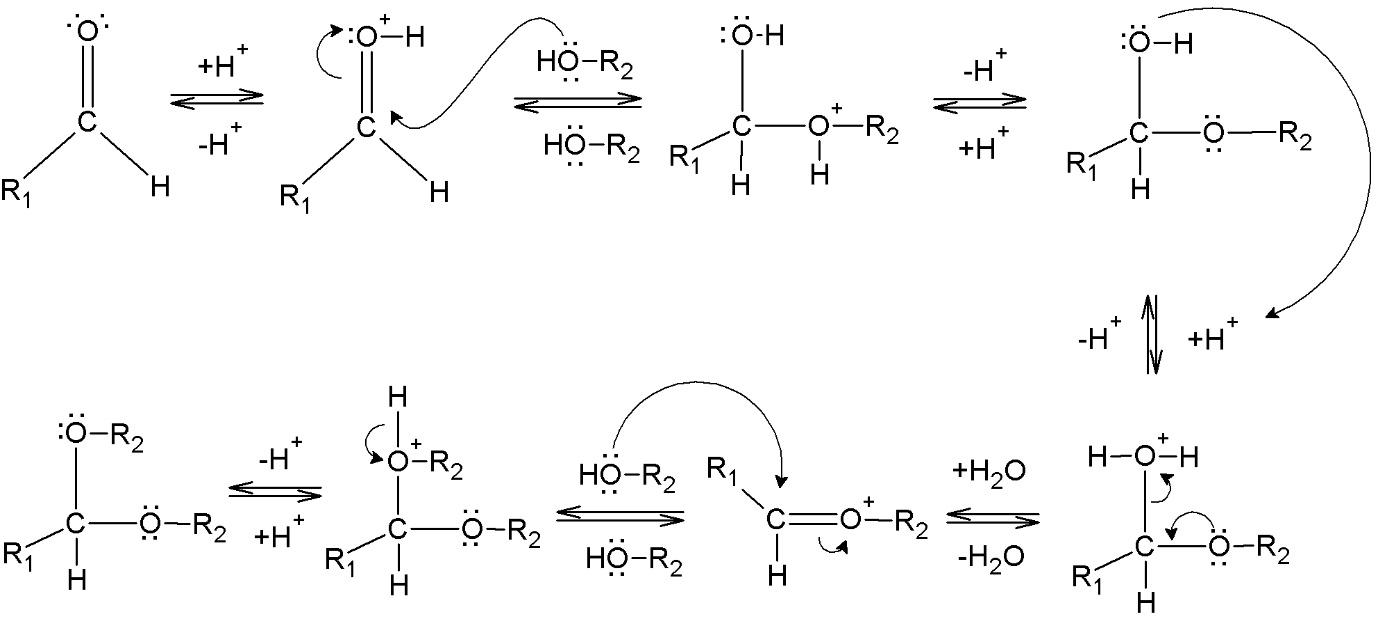
When the hydroxyl group of a hemiacetal becomes protonated and is lost as water, an acetal is formed. A molecule of alcohol quickly attacks the carbocation that has been formed. The acetal is formed when the proton from the connected alcohol is lost.
Note :
Because they are resistant against hydrolysis by bases and many oxidising and reducing agents, acetals are employed as protective groups for carbonyl groups in chemical synthesis. They can either protect a carbonyl in a molecule (by briefly reacting it with an alcohol) or they can protect a diol in a molecule (by temporarily reacting it with an alcohol) (by temporarily reacting it with a carbonyl). That is, the carbonyl, alcohols, or both might be part of the molecule whose reactivity has to be regulated.
Complete Step By Step Answer:
A functional group with the connectivity $ {R_2}C{\left( {OR'} \right)_2} $ is known as an acetal. The R groups in this case can be organic fragments (a carbon atom with various additional atoms attached) or hydrogen, but the R' groups must be organic fragments and not hydrogen. The two R' groups may or may not be equal (a "symmetric acetal") (a "mixed acetal"). Acetals are produced from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon as carbonyl compounds, but have significantly different chemical stability and reactivity. Because the centre carbon atom is saturated and possesses tetrahedral geometry, it contains four bonds.

When the hydroxyl group of a hemiacetal becomes protonated and is lost as water, an acetal is formed. A molecule of alcohol quickly attacks the carbocation that has been formed. The acetal is formed when the proton from the connected alcohol is lost.
Note :
Because they are resistant against hydrolysis by bases and many oxidising and reducing agents, acetals are employed as protective groups for carbonyl groups in chemical synthesis. They can either protect a carbonyl in a molecule (by briefly reacting it with an alcohol) or they can protect a diol in a molecule (by temporarily reacting it with an alcohol) (by temporarily reacting it with a carbonyl). That is, the carbonyl, alcohols, or both might be part of the molecule whose reactivity has to be regulated.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




