
What is the maximum number of layers of atoms in close-packed planes that will lie within two imaginary parallel planes having a distance between them as $13\sqrt{\frac{2}{3}}r$ (r is the radius of an atom) in the copper crystal (fcc)?
[ Consider the atoms to be within the parallel planes if their centres are on or within the two parallel planes.]
Answer
592.2k+ views
Hint: Unit cells are basically the smallest repeating atoms in a crystal. There are different types of unit cell like body centred unit cell represented as BCC, face centred unit cell (FCC). FCC is the face centred cubic unit cell, which is having coordination number 12 and also contains 4 atoms per unit cell.
Complete step by step answer:
- We will first draw the diagram:
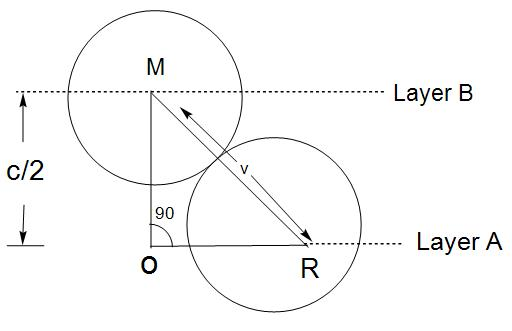
- Here, we know that the distance given in between parallel planes is $\frac{C}{2}$$\frac{C}{2}$\[\begin{align}
& \left( OM \right)+\left( RO \right)={{\left( MR \right)}^{2}} \\
& \left( \frac{C}{2} \right)+\left( \frac{2r}{\sqrt{3}} \right)={{\left( 2r \right)}^{2}} \\
\end{align}\]
- Now, by solving this equation we get :
\[\begin{align}
& {{\left( \frac{C}{2} \right)}^{2}}=\frac{8{{r}^{2}}}{3} \\
& C=4r\sqrt{\frac{2}{3}} \\
\end{align}\]
Now, we have to find the height C,
\[H(C)=4r\sqrt{\frac{2}{3}}\]
- We can also write this equation as:
\[or,\text{ }H(C)=2a\sqrt{\frac{2}{3}}\]
- Now, the distance between two layers that is A and B= $\frac{C}{2}=\sqrt{\frac{8}{3}}(r)=\sqrt{\frac{2}{3}}(2r)$
- The given distance between two imaginary plane=$13\sqrt{\frac{2}{3}}(r)$
Now, let K number of imaginary planes=
\[\begin{align}
& K\times \frac{\sqrt{2}}{\sqrt{3}}\left( r \right) \\
& =13\sqrt{\frac{2}{3}}\left( r \right) \\
& K\approx 6.5 \\
& K=7 \\
\end{align}\]
\[\]
Hence, we can conclude that the maximum number of layers of atoms in close-packed planes will be 7.
Note: We must not get confused in terms of FCC and BCC. FCC is face centred cubic unit cell, it contains four atoms per unit cell. Whereas, BCC is a body centred unit cell, it contains 2 atoms per unit cell.
Complete step by step answer:
- We will first draw the diagram:

- Here, we know that the distance given in between parallel planes is $\frac{C}{2}$$\frac{C}{2}$\[\begin{align}
& \left( OM \right)+\left( RO \right)={{\left( MR \right)}^{2}} \\
& \left( \frac{C}{2} \right)+\left( \frac{2r}{\sqrt{3}} \right)={{\left( 2r \right)}^{2}} \\
\end{align}\]
- Now, by solving this equation we get :
\[\begin{align}
& {{\left( \frac{C}{2} \right)}^{2}}=\frac{8{{r}^{2}}}{3} \\
& C=4r\sqrt{\frac{2}{3}} \\
\end{align}\]
Now, we have to find the height C,
\[H(C)=4r\sqrt{\frac{2}{3}}\]
- We can also write this equation as:
\[or,\text{ }H(C)=2a\sqrt{\frac{2}{3}}\]
- Now, the distance between two layers that is A and B= $\frac{C}{2}=\sqrt{\frac{8}{3}}(r)=\sqrt{\frac{2}{3}}(2r)$
- The given distance between two imaginary plane=$13\sqrt{\frac{2}{3}}(r)$
Now, let K number of imaginary planes=
\[\begin{align}
& K\times \frac{\sqrt{2}}{\sqrt{3}}\left( r \right) \\
& =13\sqrt{\frac{2}{3}}\left( r \right) \\
& K\approx 6.5 \\
& K=7 \\
\end{align}\]
\[\]
Hence, we can conclude that the maximum number of layers of atoms in close-packed planes will be 7.
Note: We must not get confused in terms of FCC and BCC. FCC is face centred cubic unit cell, it contains four atoms per unit cell. Whereas, BCC is a body centred unit cell, it contains 2 atoms per unit cell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




